Label: ALTRIXA- vitamin tablet, coated
- NHRIC Code(s): 28595-715-30
- Packager: Allegis Pharmaceuticals, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated March 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
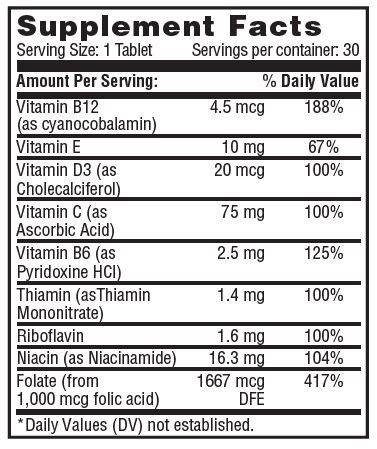
Suplement FactsOther Ingredients: cellulose, vegetable stearic acid, silica, croscarmellose sodium, vegetable megnesium stearate, cellulose coating, titanium oxide.
-
DescriptionAltrixa Tablets is a prescription dietary supplement intended for oral administration. Altrixa Tablets is to provide significant amounts of Vitamins B6, B12, C, D3, E, riboflavin, niacinamide ...
-
WarningsKEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a licensed medical practitioner or poison control center immediately. For use under the supervision of ...
-
Doseage and AdministrationUsual adult dose is one tablet daily, or as prescribed by a licensed medical practitioner. How Supplied - Altrix Tablets are avaialble as white, round tablets with AL07 imprint and are available ...
-
StorageStore at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.] Protect from light and moisture.Tamper Evident: Do not use if seal if ...
-
Label

-
INGREDIENTS AND APPEARANCEProduct Information