Label: GUANFACINE tablet
- NDC Code(s): 27241-242-01, 27241-243-01
- Packager: Ajanta Pharma USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
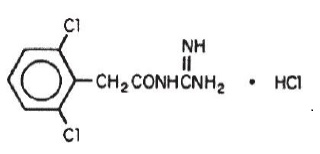
DESCRIPTIONGuanfacine hydrochloride, USP is a centrally acting antihypertensive with α2-adrenoceptor agonist properties in tablet form for oral administration. The chemical name of guanfacine hydrochloride ...
-
CLINICAL PHARMACOLOGYGuanfacine hydrochloride is an orally active antihypertensive agent whose principal mechanism of action appears to be stimulation of central α2-adrenergic receptors. By stimulating these ...
-
INDICATIONS AND USAGEGuanfacine tablets, USP are indicated in the management of hypertension. Guanfacine tablets, USP may be given alone or in combination with other antihypertensive agents, especially thiazide-type ...
-
CONTRAINDICATIONSGuanfacine tablets are contraindicated in patients with known hypersensitivity to guanfacine hydrochloride.
-
PRECAUTIONSGeneral - Like other antihypertensive agents, guanfacine hydrochloride should be used with caution in patients with severe coronary insufficiency, recent myocardial infarction, cerebrovascular ...
-
ADVERSE REACTIONSAdverse reactions noted with guanfacine hydrochloride are similar to those of other drugs of the central α2-adrenoreceptor agonist class: dry mouth, sedation (somnolence), weakness (asthenia) ...
-
DRUG ABUSE AND DEPENDENCENo reported abuse or dependence has been associated with the administration of guanfacine hydrochloride. OVERDOSAGE - Signs and Symptoms - Drowsiness, lethargy, bradycardia and hypotension ...
-
DOSAGE AND ADMINISTRATIONThe recommended initial dose of guanfacine tablets when given alone or in combination with another antihypertensive drug is 1 mg daily given at bedtime to minimize somnolence. If after 3 weeks to ...
-
HOW SUPPLIEDGuanfacine tablets, USP are available in the following dosing strengths (expressed in equivalent amounts of guanfacine): 1 mg—White to off white, round, biconvex tablets, debossed with ‘G1’ on ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 27241-242-01 - 100 Tablets - Guanfacine Tablets, USP - 1 mg - Rx Only - ajanta - NDC 27241-243-01 - 100 Tablets - Guanfacine Tablets, USP - 2 mg - Rx Only - ajanta
-
INGREDIENTS AND APPEARANCEProduct Information