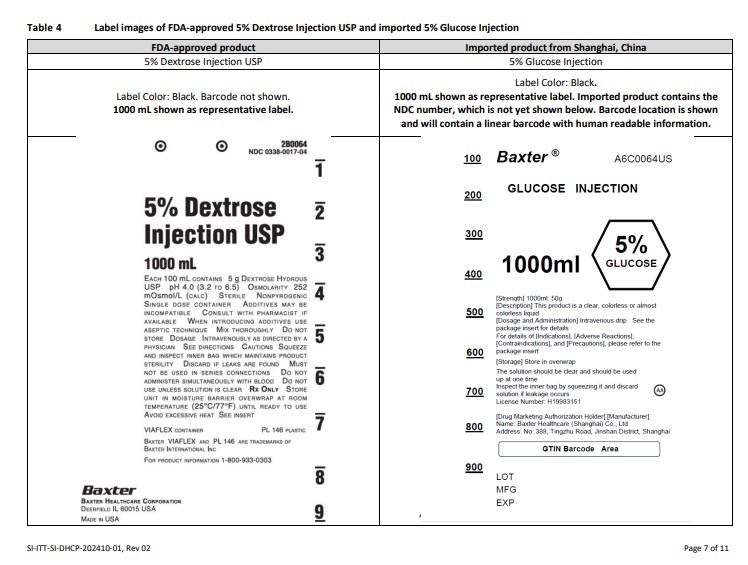
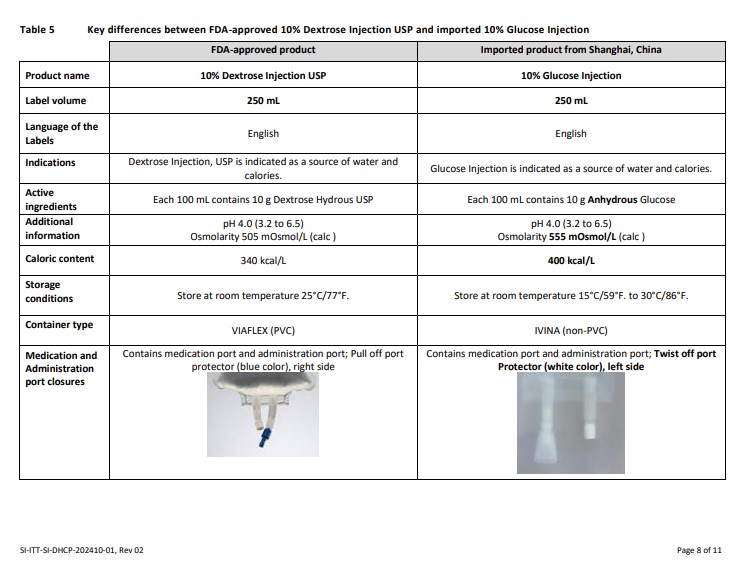
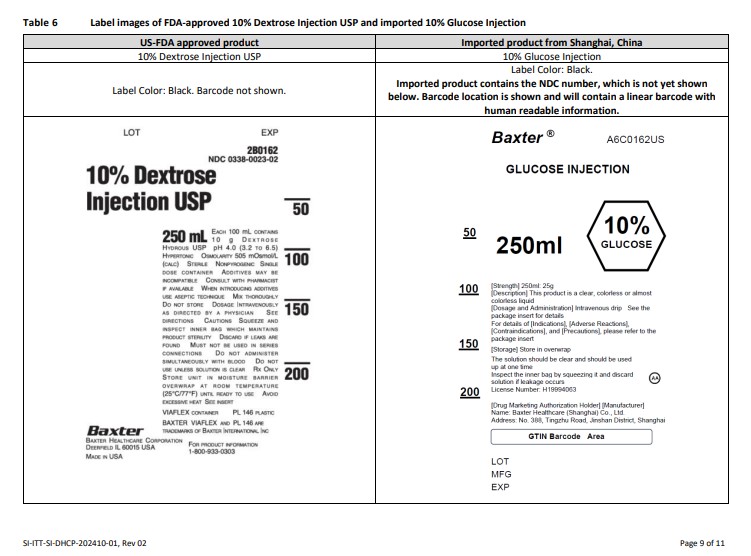
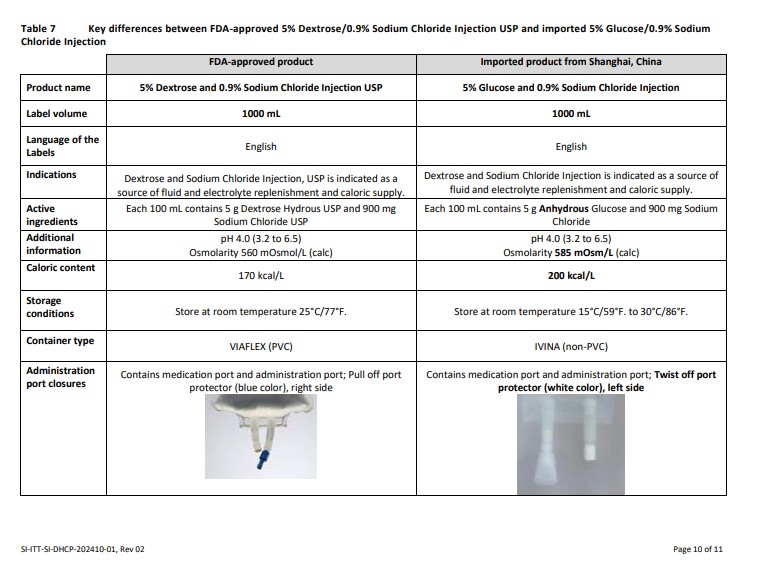
Label: GLUCOSE- dextrose anhydrous injection, solution
- NDC Code(s): 0338-9795-01, 0338-9795-40, 0338-9801-01, 0338-9801-12
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CARE PROFESSIONAL LETTER
Reporting Adverse Events or Product Quality Issues
To report adverse events associated with these imported products, please call Baxter at 1-866-888-2472, or fax: 1- 800-759-1801. Adverse events or quality problems experienced with the use of these imported products may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
• Complete and submit the report Online: www.fda.gov/medwatch/report.htm
• Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.To report product quality issues associated with these imported products, please contact Baxter Product Surveillance through Baxter - Product Feedback Portal (https://productfeedback.baxter.com/).
Please also refer to the local prescribing information of the imported product, translated into English, available for:
• 0.9% Sodium Chloride Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723233)
• 5% Glucose Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723235)
• 10% Glucose Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723237)
• 5% Glucose/0.9% Sodium Chloride Injection (click https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/723238)Please refer to the FDA-approved prescribing information for each drug product listed below:
• 0.9% Sodium Chloride Injection USP (click https://www.dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=f55bd888-5e01-474d-871b-24654c070178&type=pdf&name=f55bd888-5e01-474d-871b-24654c070178)
• 5% Dextrose Injection USP (click https://www.dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=3bb406a9-f5cb-403a-b1bb-5c4facbea3d5&type=pdf&name=3bb406a9-f5cb-403a-b1bb-5c4facbea3d5)
• 10% Dextrose Injection USP (click https://www.dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=3bb406a9-f5cb-403a-b1bb-5c4facbea3d5&type=pdf&name=3bb406a9-f5cb-403a-b1bb-5c4facbea3d5)
• 5% Dextrose/0.9% Sodium Chloride Injection USP (click https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/016678s007,016683s103,016687s104,016689s107,016697s098lbl.pdf) - PACKAGE INSERT
-
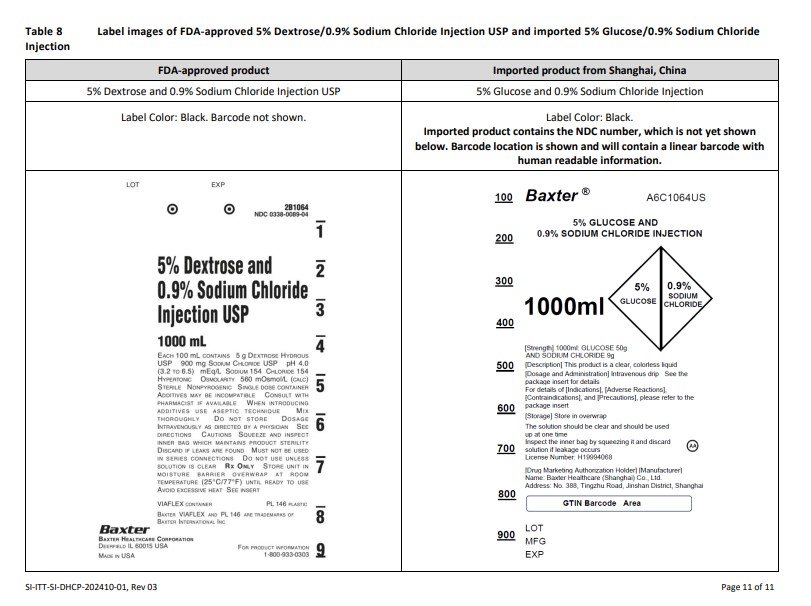
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Baxter Logo Trademark
A6C0062US
GLUCOSE INJECTION
50
100
150
200
250ml
5% GLUCOSE[Strength] 250ml: 12.5g
[Description] This product is a colorless or almost
colorless clear liquid
[Dosage and Administration] Intravenous drip See the
package insert for details
For details of [Indications], [Adverse Reactions],
[Contraindications], and [Precautions], please refer to the
package insert[Storage] Store in overwrap
The solution should be clear and should be used
up at one time
Inspect the inner bag by squeezing it and discard
solution if leakage occurs
License Number: H19994071AA
[Drug Marketing Authorization Holder] [Manufacturer]
Name: Baxter Healthcare (Shanghai) Co., Ltd.
Address: No. 388, Tingzhu Road, Jinshan District, ShanghaiBarCode
(01) 00303389795018LOT
MFG
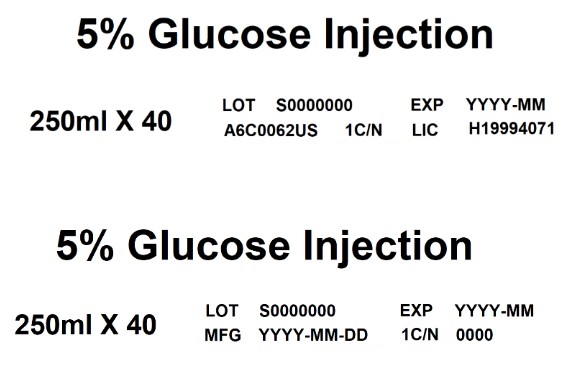
EXP5% Glucose Injection
250ml X 40
LOT S0000000 EXP YYYY-MM
A6C0062US 1C/N LIC H199940715% Glucose Injection
250ml X 40
LOT S0000000 EXP YYYY-MM
MFG YYYY-MM-DD 1C/N 0000Baxter Logo Trademark
A6C0064US
GLUCOSE INJECTION
100
200
300
400
500
600
700
800
900
1000ml
5% GLUCOSE[Strength] 1000ml: 50g
[Description] This product is a colorless or almost
colorless clear liquid
[Dosage and Administration] Intravenous drip See the
package insert for details
For details of [Indications], [Adverse Reactions],
[Contraindications], and [Precautions], please refer to the
package insert[Storage] Store in overwrap
The solution should be clear and should be used
up at one time
Inspect the inner bag by squeezing it and discard
solution if leakage occurs
License Number: H19983151AA
[Drug Marketing Authorization Holder] [Manufacturer]
Name: Baxter Healthcare (Shanghai) Co., Ltd.
Address: No. 388, Tingzhu Road, Jinshan District, ShanghaiBarCode
(01) 00303389801016LOT
MFG
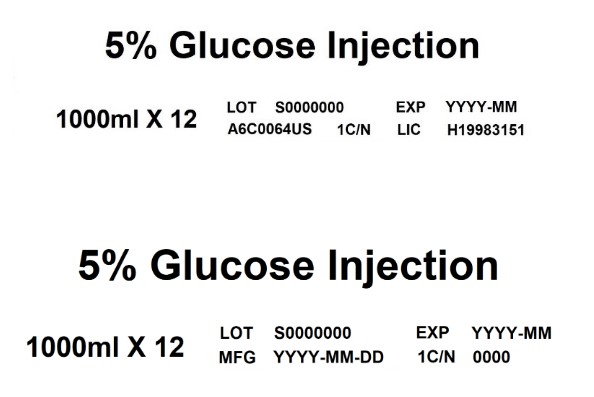
EXP5% Glucose Injection
1000ml X 12
LOT S0000000 EXP YYYY-MM
A6C0064US 1C/N LIC H199831515% Glucose Injection
1000ml X 12
LOT S0000000 EXP YYYY-MM
MFG YYYY-MM-DD 1C/N 0000 -
INGREDIENTS AND APPEARANCE
GLUCOSE
dextrose anhydrous injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9795 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 55 g in 1000 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9795-40 40 in 1 CARTON 10/10/2024 1 NDC:0338-9795-01 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/10/2024 GLUCOSE
dextrose anhydrous injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-9801 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 55 g in 1000 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9801-12 12 in 1 CARTON 10/10/2024 1 NDC:0338-9801-01 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/10/2024 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare (Shanghai) Co. Ltd. 527191860 MANUFACTURE(0338-9795, 0338-9801) , ANALYSIS(0338-9795, 0338-9801) , LABEL(0338-9795, 0338-9801) , PACK(0338-9795, 0338-9801) , STERILIZE(0338-9795, 0338-9801)