Label: TYVASO DPI- treprostinil inhalant
TYVASO DPI- treprostinil kit
- NDC Code(s): 66302-600-02, 66302-610-02, 66302-616-03, 66302-620-03, view more
- Packager: United Therapeutics Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TYVASO DPI safely and effectively. See full prescribing information for TYVASO DPI. TYVASO DPI® (treprostinil) inhalation powder ...
-
Table of ContentsTable of Contents
-
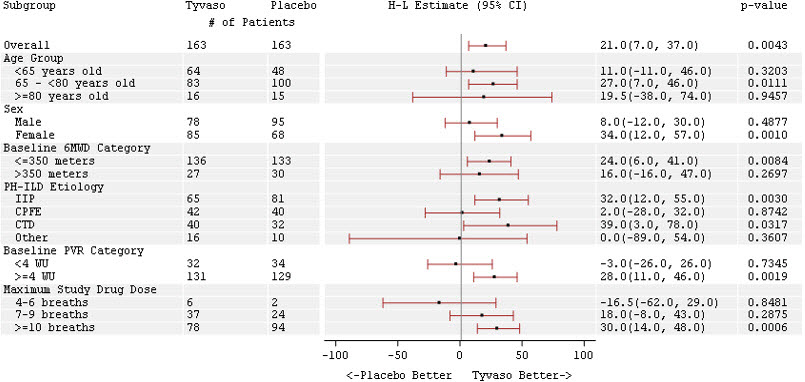
1 INDICATIONS AND USAGE1.1 Pulmonary Arterial Hypertension - Tyvaso DPI is indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies with Tyvaso establishing ...
-
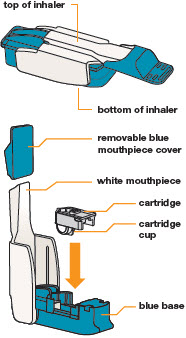
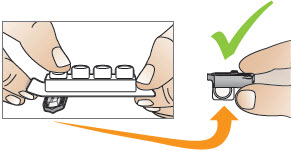
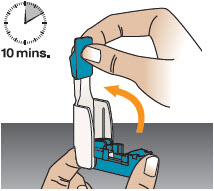
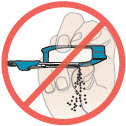
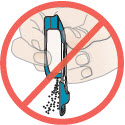
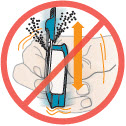
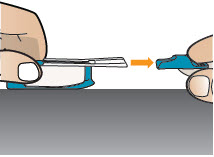
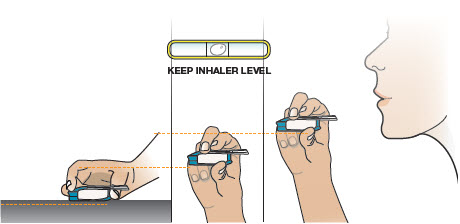
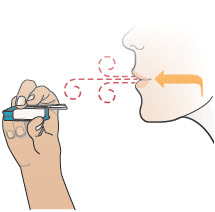
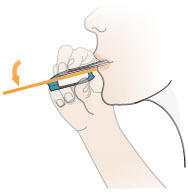
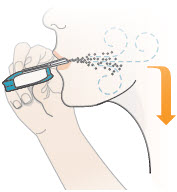
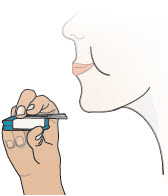
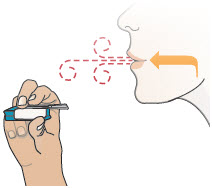
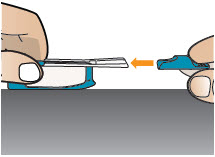
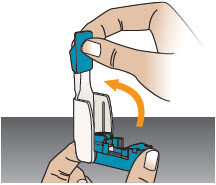
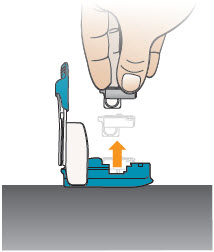
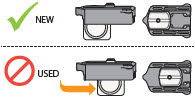
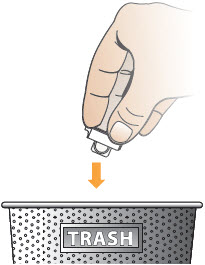
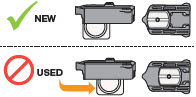
2 DOSAGE AND ADMINISTRATION2.1 Administration - Use Tyvaso DPI only with the Tyvaso DPI Inhaler. Tyvaso DPI is administered using a single inhalation per cartridge. Administer Tyvaso DPI in 4 separate, equally spaced ...
-
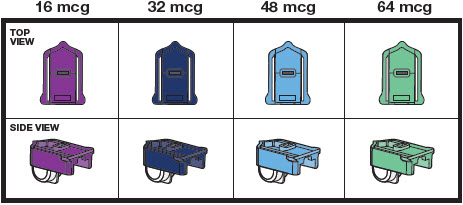
3 DOSAGE FORMS AND STRENGTHSInhalation powder: Single-dose plastic cartridges containing 16 mcg, 32 mcg, 48 mcg, 64 mcg, or 80 mcg of treprostinil as a dry powder formulation.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Symptomatic Hypotension - Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Tyvaso DPI may produce symptomatic ...
-
6 ADVERSE REACTIONSThe following potential adverse reactions are described in Warnings and Precautions (5): - Decrease in systemic blood pressure [see Warnings and Precautions (5.1)]. - Bleeding [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Bosentan - In a human pharmacokinetic study conducted with bosentan (250 mg/day) and an oral formulation of treprostinil (treprostinil diolamine), no pharmacokinetic interactions between ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, there ...
-
10 OVERDOSAGEIn general, symptoms of overdose with inhaled treprostinil include flushing, headache, hypotension, nausea, vomiting, and diarrhea. Provide general supportive care until the symptoms of overdose ...
-
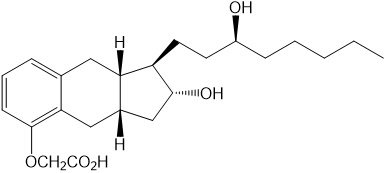
11 DESCRIPTION11.1 Tyvaso DPI Cartridges - Tyvaso DPI consists of single-dose plastic cartridges filled with a white powder containing 1% of treprostinil, a prostacyclin mimetic, which is intended for ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Treprostinil is a prostacyclin analogue. The major pharmacologic actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A 2-year rat carcinogenicity study was performed with treprostinil inhalation at target doses of 5.26, 10.6, and 34.1 mcg/kg/day. There ...
-
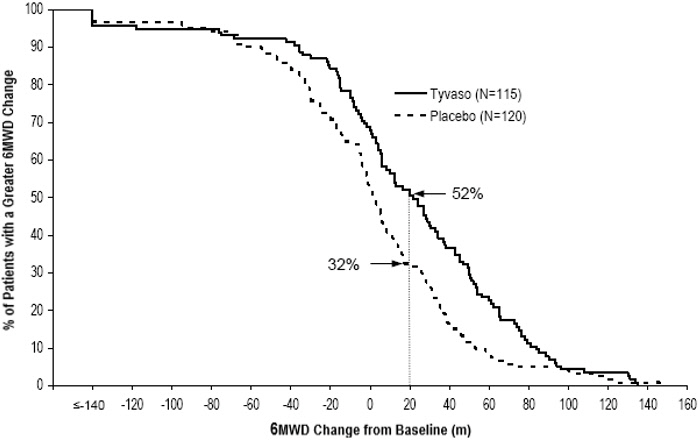
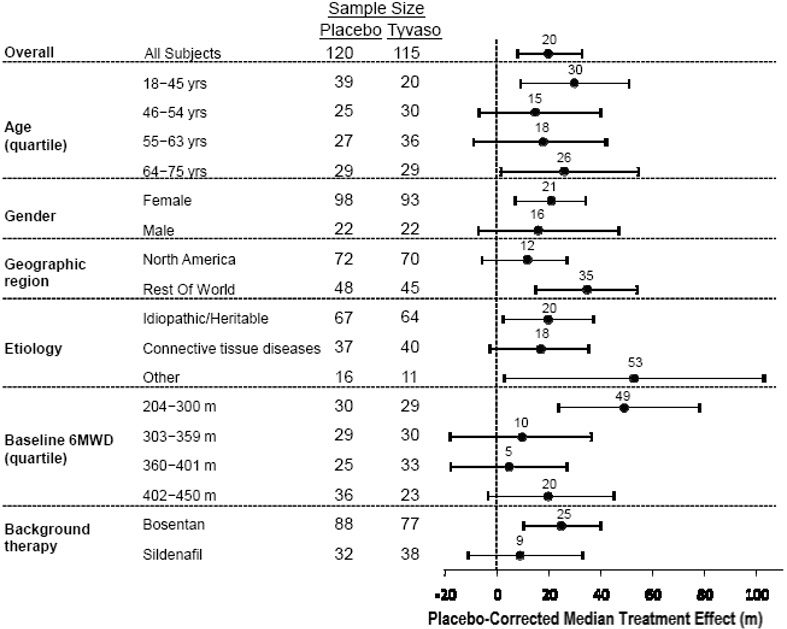
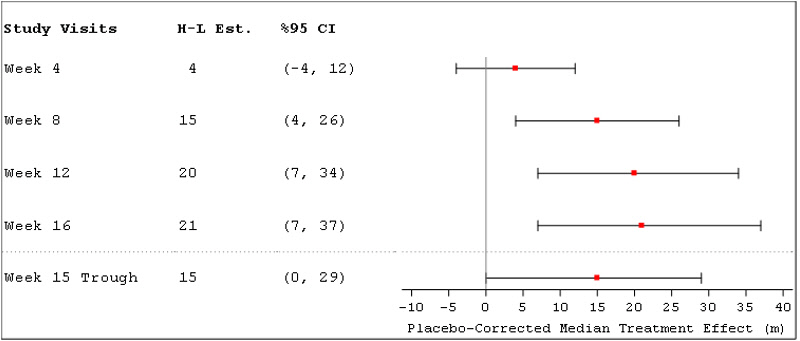
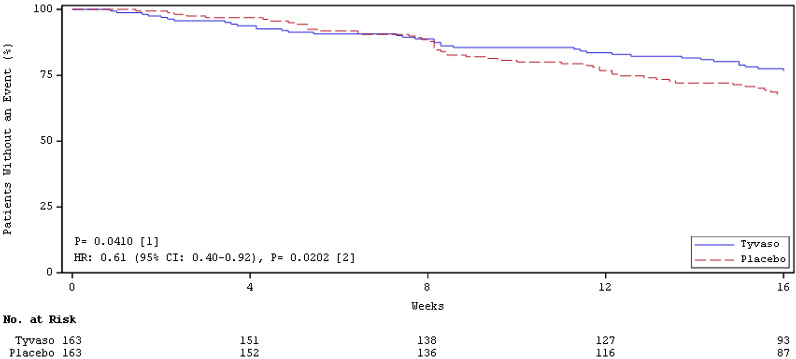
14 CLINICAL STUDIES14.1 Pulmonary Arterial Hypertension (WHO Group 1) (TRIUMPH I) TRIUMPH I, was a 12-week, randomized, double-blind, placebo-controlled, multicenter study of patients with PAH (NCT00147199). The ...
-
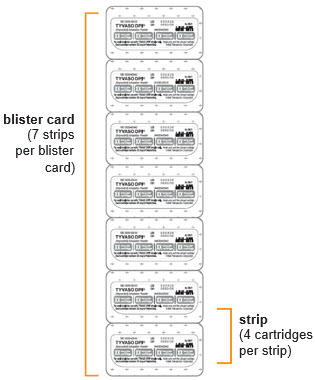
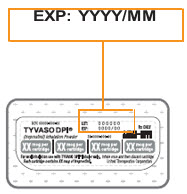
16 HOW SUPPLIED/STORAGE AND HANDLINGTyvaso DPI (treprostinil) inhalation powder is available as 16 mcg, 32 mcg, 48 mcg, 64 mcg, or 80 mcg of treprostinil in single-dose plastic cartridges with approximate fill weights of 1.6 mg, 3.2 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Train patients in the administration process for Tyvaso DPI, including dosing, Tyvaso DPI Inhaler setup ...
-
SPL UNCLASSIFIED SECTION©Copyright 2024 United Therapeutics Corp. All rights reserved. Tyvaso DPI® and Tyvaso® are registered trademarks of United Therapeutics Corporation. Tyvaso DPI manufactured by: MannKind ...
-
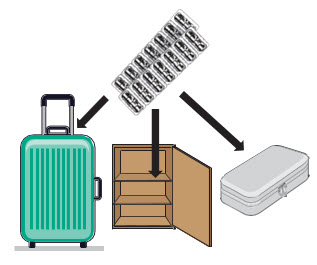
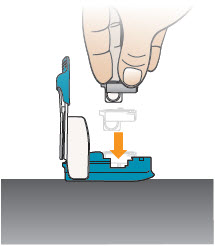
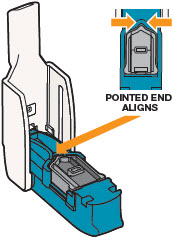
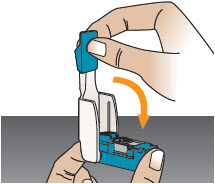
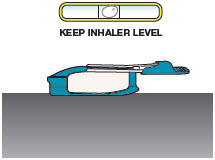
INSTRUCTIONS FOR USEInstructions - for Use - TYVASO [ tī-vā'-sō ] DPI® (treprostinil) Inhalation Powder - For oral inhalation only - Table of Contents - page(s) Read Before Starting1 - Important ...
-
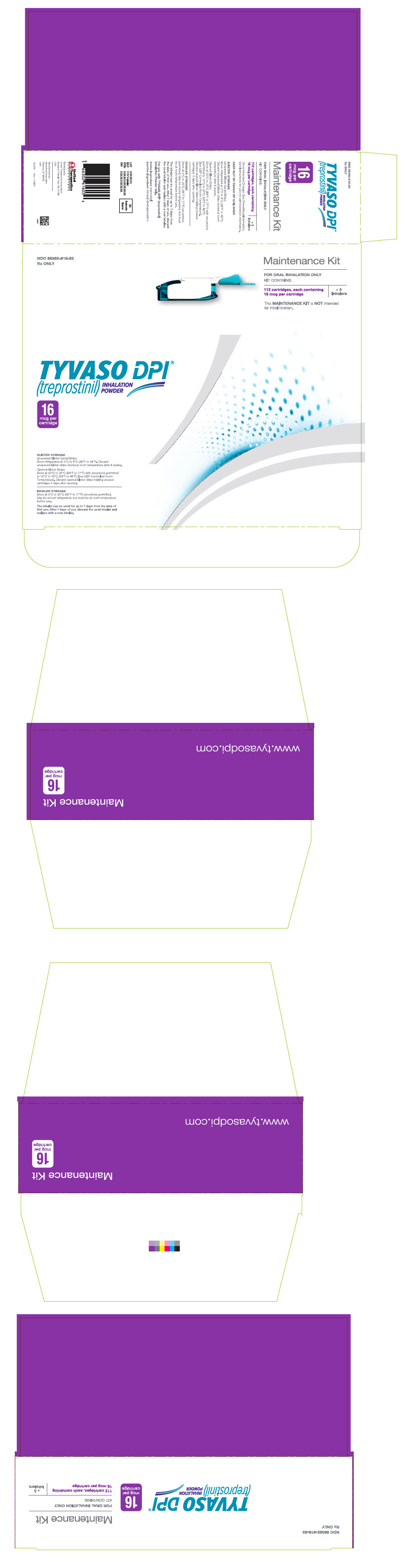
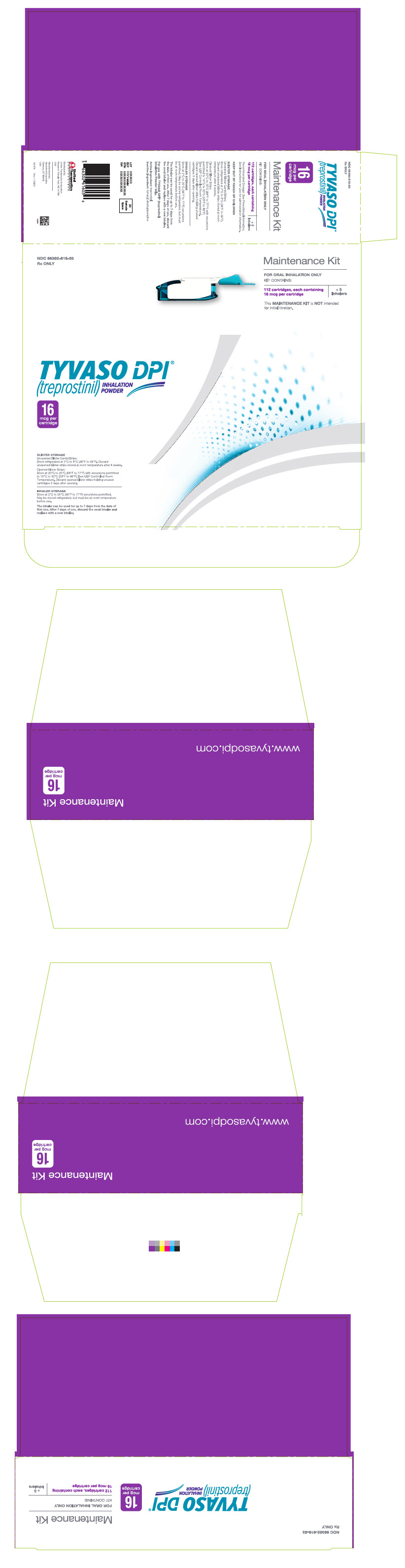
PRINCIPAL DISPLAY PANEL - 16 mcg Maintenance KitNDC 66302-616-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 16 mcg per cartridge - + 5 - Inhalers - This MAINTENANCE KIT is NOT intended - for ...
-
PRINCIPAL DISPLAY PANEL - 16 mcg Institutional KitNDC 66302-716-04 - Rx ONLY - Institutional Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 16 cartridges, each containing - 16 mcg per cartridge - + 2 - Inhalers - This INSTITUTIONAL KIT is NOT intended - for ...
-
PRINCIPAL DISPLAY PANEL - 32 mcg Maintenance KitNDC 66302-632-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 32 mcg per cartridge - + 5 - Inhalers - This MAINTENANCE KIT is NOT intended - for ...
-
PRINCIPAL DISPLAY PANEL - 32 mcg Institutional KitNDC 66302-732-04 - Rx ONLY - Institutional Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 16 cartridges, each containing - 32 mcg per cartridge - + 2 - Inhalers - This INSTITUTIONAL KIT is NOT intended - for ...
-
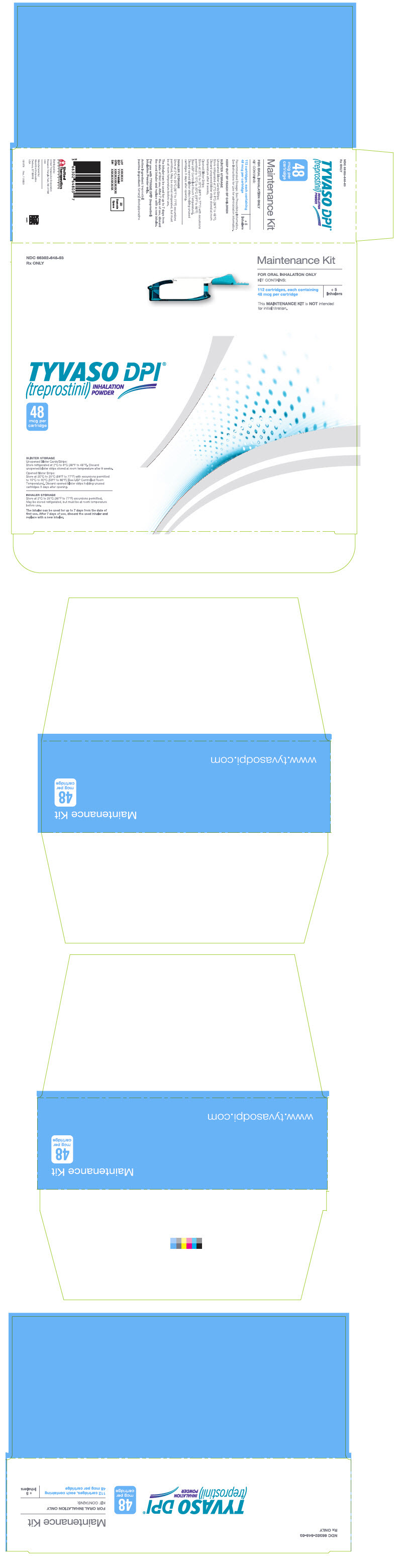
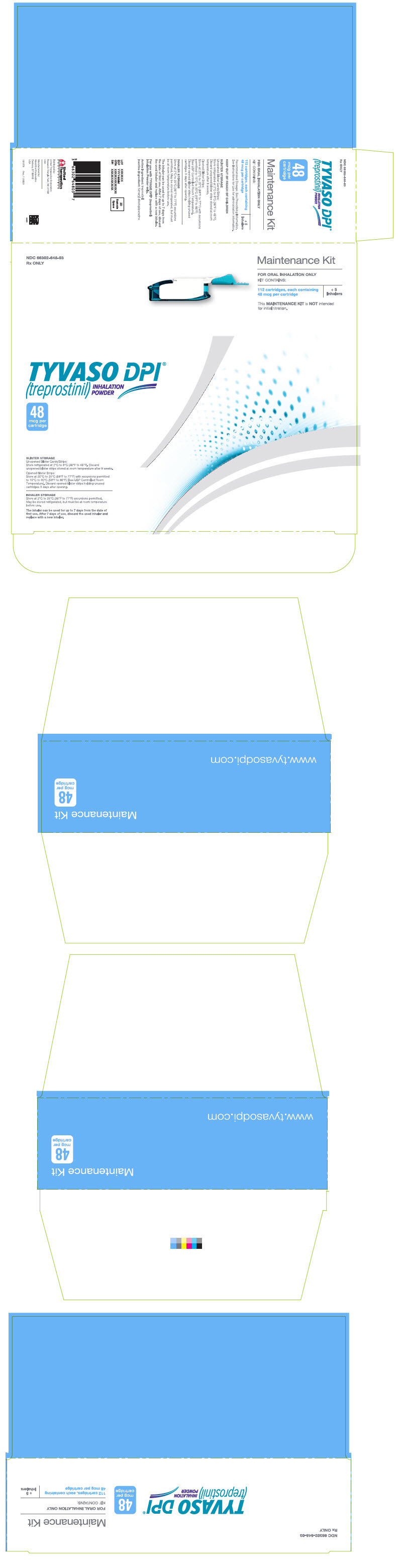
PRINCIPAL DISPLAY PANEL - 48 mcg Maintenance KitNDC 66302-648-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 48 mcg per cartridge - + 5 - Inhalers - This MAINTENANCE KIT is NOT intended - for ...
-
PRINCIPAL DISPLAY PANEL - 48 mcg Institutional KitNDC 66302-748-04 - Rx ONLY - Institutional Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 16 cartridges, each containing - 48 mcg per cartridge - + 2 - Inhalers - This INSTITUTIONAL KIT is NOT intended - for ...
-
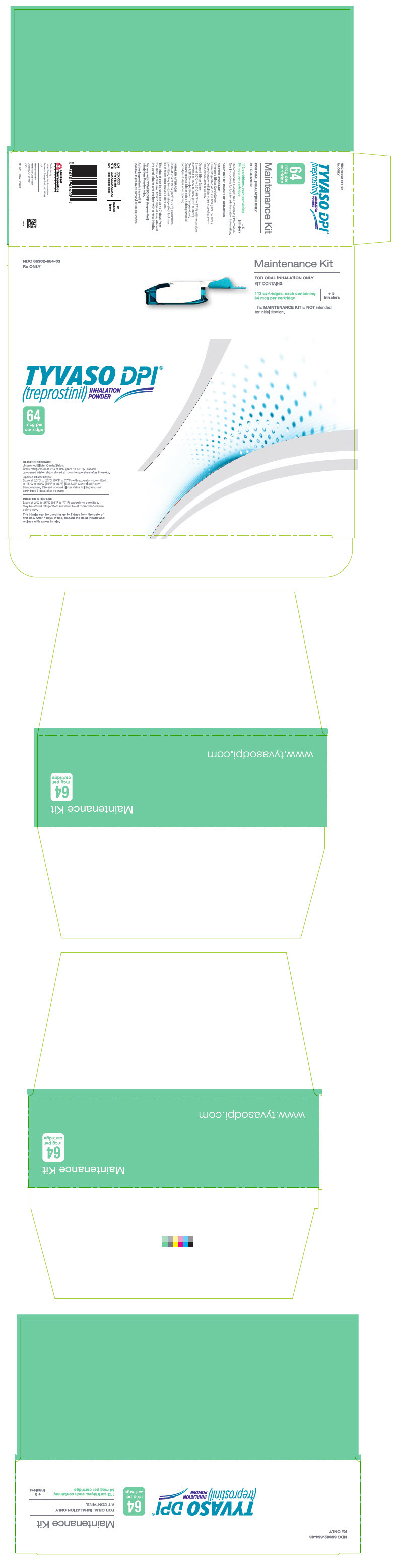
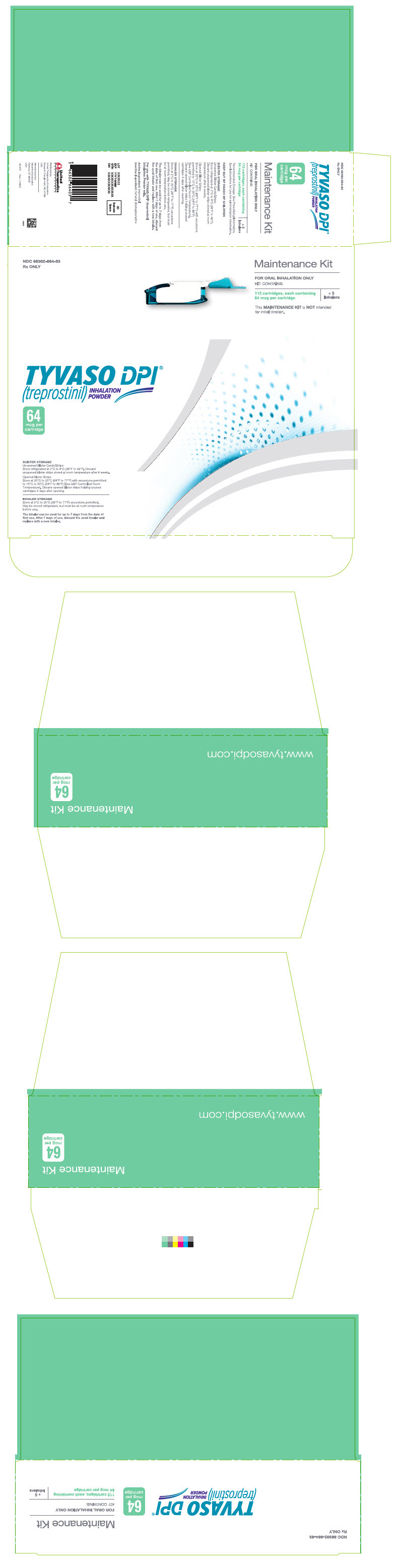
PRINCIPAL DISPLAY PANEL - 64 mcg Maintenance KitNDC 66302-664-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 64 mcg per cartridge - + 5 - Inhalers - This MAINTENANCE KIT is NOT intended - for ...
-
PRINCIPAL DISPLAY PANEL - 64 mcg Institutional KitNDC 66302-764-04 - Rx ONLY - Institutional Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 16 cartridges, each containing - 64 mcg per cartridge - + 2 - Inhalers - This INSTITUTIONAL KIT is NOT intended - for ...
-
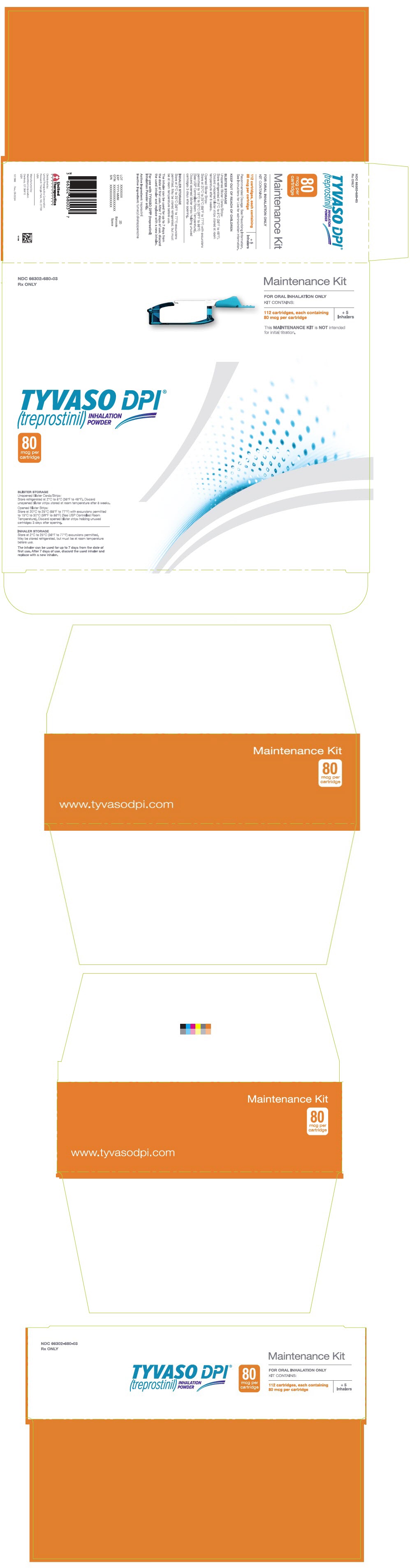
PRINCIPAL DISPLAY PANEL - 80 mcg Maintenance KitNDC 66302-680-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 80 mcg per cartridge - + 5 - Inhalers - This MAINTENANCE KIT is NOT intended - for initial ...
-
PRINCIPAL DISPLAY PANEL - 80 mcg Institutional KitNDC 66302-780-04 - Rx ONLY - Institutional Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 16 cartridges, each containing - 80 mcg per cartridge - + 2 - Inhalers - This INSTITUTIONAL KIT is NOT intended - for ...
-
PRINCIPAL DISPLAY PANEL - 16 mcg 32 mcg Titration KitNDC 66302-600-02 - Rx ONLY - Titration Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 16 mcg per cartridge - 84 cartridges, each containing - 32 mcg per cartridge - + 5 ...
-
PRINCIPAL DISPLAY PANEL - 32 mcg 48 mcg Maintenance KitNDC 66302-620-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 32 mcg per cartridge - 112 cartridges, each containing - 48 mcg per cartridge - + 5 ...
-
PRINCIPAL DISPLAY PANEL - 32 mcg 64 mcg Maintenance KitNDC 66302-630-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 32 mcg per cartridge - 112 cartridges, each containing - 64 mcg per cartridge - + 5 ...
-
PRINCIPAL DISPLAY PANEL - 48 mcg 64 mcg Maintenance KitNDC 66302-640-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 48 mcg per cartridge - 112 cartridges, each containing - 64 mcg per cartridge - ...
-
PRINCIPAL DISPLAY PANEL - 32 mcg 48 mcg Institutional KitNDC 66302-720-04 - Rx ONLY - Institutional Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 16 cartridges, each containing - 32 mcg per cartridge - 16 cartridges, each containing - 48 mcg per cartridge - + 2 ...
-
PRINCIPAL DISPLAY PANEL - 16 mcg 32 mcg 48 mcg Titration KitNDC 66302-610-02 - Rx ONLY - Titration Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 16 mcg per cartridge - 112 cartridges, each containing - 32 mcg per cartridge - 28 ...
-
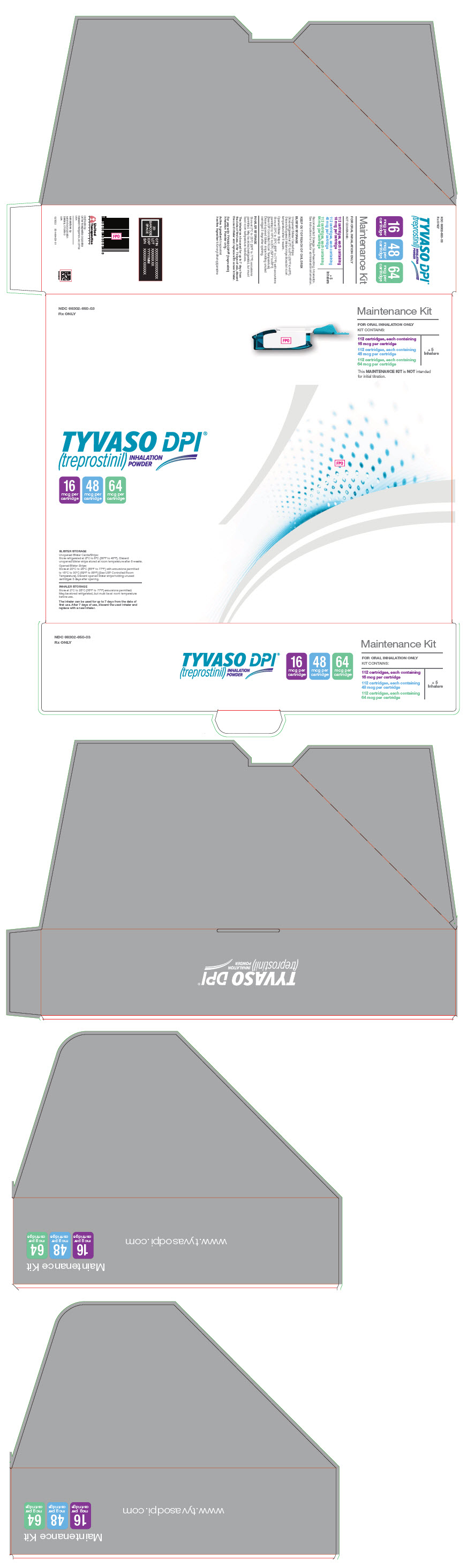
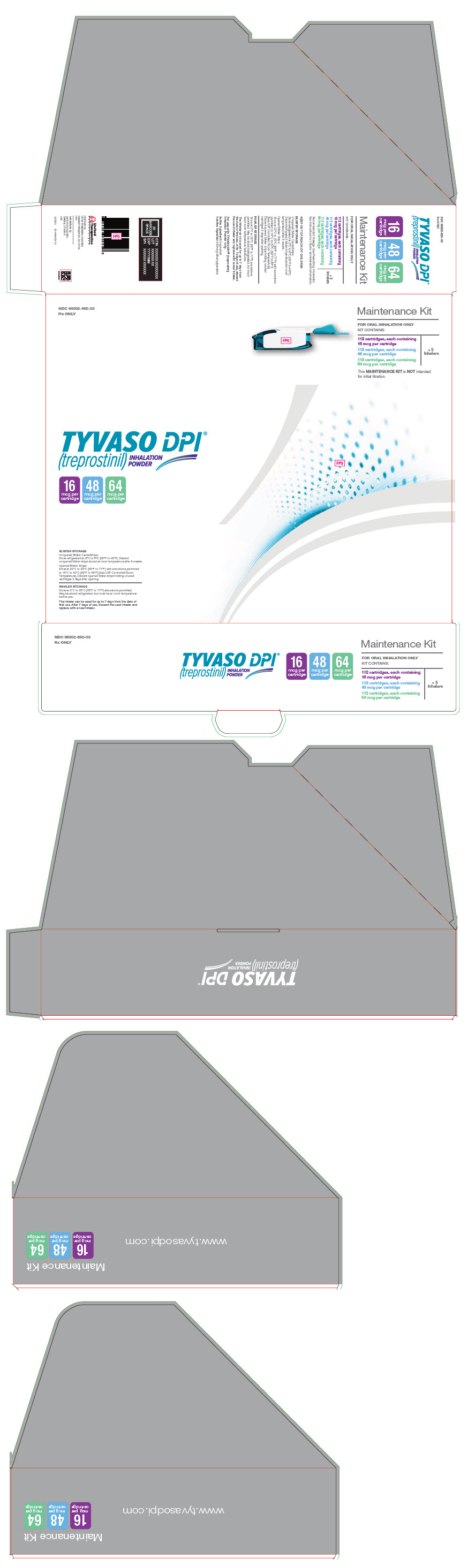
PRINCIPAL DISPLAY PANEL - 16 mcg 48 mcg 64 mcg Maintenance KitNDC 66302-650-03 - Rx ONLY - Maintenance Kit - FOR ORAL INHALATION ONLY - KIT CONTAINS: 112 cartridges, each containing - 16 mcg per cartridge - 112 cartridges, each containing - 48 mcg per cartridge - 112 ...
-
INGREDIENTS AND APPEARANCEProduct Information