Label: SEVOSPIRE- sevoflurane liquid
- NDC Code(s): 17033-092-25
- Packager: Dechra Veterinary Products LLC
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
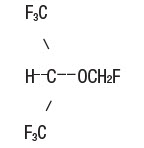
Sevospire (sevoflurane), a volatile liquid, is a halogenated general inhalation anesthetic drug. Its chemical name is fluoromethyl 2,2,2-trifluoro-1 (trifluoromethyl) ethyl ether, and its structural formula is:
Sevoflurane Physical Constants are:
Molecular weight 200.05 Boiling point at 760 mm Hg 58.6°C Specific gravity at 20°C 1.520-1.525 g/mL Vapor pressure in mm Hg at 20°C 157 at 25°C 197 at 36°C 317 Distribution Partition Coefficients at 37°C:
Blood/Gas 0.63-0.69 Water/Gas 0.36 Olive Oil/Gas 47-54 Brain/Gas 1.15 Mean Component/Gas Partition Coefficients at 25°C for Polymers Used Commonly in Medical Applications:
Conductive rubber 14.0 Butyl rubber 7.7 Polyvinyl chloride 17.4 Polyethylene 1.3 Sevoflurane is nonflammable and nonexplosive as defined by the requirements of International Electrotechnical Commission 601-2-13.
Sevoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers.
Sevoflurane is nonpungent. It is miscible with ethanol, ether, chloroform and petroleum benzene, and it is slightly soluble in water. Sevoflurane is stable when stored under normal room lighting condition according to instructions.
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
Inspired Concentration: The delivered concentration of sevoflurane should be known. Since the depth of anesthesia may be altered easily and rapidly, only vaporizers producing predictable percentage concentrations of sevoflurane should be used. Sevoflurane should be vaporized using a precision vaporizer specifically calibrated for sevoflurane. Sevoflurane contains no stabilizer. Nothing in the drug product alters calibration or operation of these vaporizers. The administration of general anesthesia must be individualized based on the patient's response.
WHEN USING SEVOFLURANE, PATIENTS SHOULD BE CONTINUOUSLY MONITORED AND FACILITIES FOR MAINTENANCE OF PATENT AIRWAY, ARTIFICIAL VENTILATION, AND OXYGEN SUPPLEMENTATION MUST BE IMMEDIATELY AVAILABLE.
Replacement of Desiccated CO2 Absorbents: When a clinician suspects that the CO2 absorbent may be desiccated, it should be replaced. An exothermic reaction occurs when sevoflurane is exposed to CO2 absorbents. This reaction is increased when the CO2 absorbent becomes desiccated (see PRECAUTIONS).
Premedication: No specific premedication is either indicated or contraindicated with sevoflurane. The necessity for and choice of premedication is left to the discretion of the veterinarian. Preanesthetic doses for premedicants may be lower than the label directions for their use as a single medication.1
Induction: For mask induction using sevoflurane alone, inspired concentrations of up to 7% sevoflurane with oxygen are employed to induce surgical anesthesia in the healthy dog. These concentrations can be expected to produce surgical anesthesia in 3 to 14 minutes. Due to the rapid and dose dependent changes in anesthetic depth, care should be taken to prevent overdosing. Respiration must be monitored closely in the dog and supported when necessary with supplemental oxygen and/or assisted ventilation.
Maintenance: Sevospire may be used for maintenance anesthesia following mask induction using sevoflurane or following injectable induction agents. The concentration of vapor necessary to maintain anesthesia is much less than that required to induce it.
Surgical levels of anesthesia in the healthy dog may be maintained with inhaled concentrations of 3.7-4.0% sevoflurane in oxygen in the absence of premedication and 3.3-3.6% in the presence of premedication. The use of injectable induction agents without premedication has little effect on the concentrations of sevoflurane required for maintenance.
Anesthetic regimens that include opioid, alpha2 -agonist, benzodiazepine or phenothiazine premedication will allow the use of lower sevoflurane maintenance concentrations.
- CONTRAINDICATIONS
-
WARNINGS
Sevoflurane is a profound respiratory depressant.
DUE TO THE RAPID AND DOSE DEPENDENT CHANGES IN ANESTHETIC DEPTH, RESPIRATION MUST BE MONITORED CLOSELY IN THE DOG AND SUPPORTED WHEN NECESSARY WITH SUPPLEMENTAL OXYGEN AND/OR ASSISTED VENTILATION.
In cases of severe cardiopulmonary depression, discontinue drug administration, ensure the existence of a patent airway and initiate assisted or controlled ventilation with pure oxygen. Cardiovascular depression should be treated with plasma expanders, pressor agents, antiarrhythmic agents or other techniques as appropriate for the observed abnormality.
Due to sevoflurane's low solubility in blood, increasing the concentration may result in rapid changes in anesthetic depth and hemodynamic changes (dose dependent decreases in respiratory rate and blood pressure) compared to other volatile anesthetics. Excessive decreases in blood pressure or respiratory depression may be corrected by decreasing or discontinuing the inspired concentration of sevoflurane.
Potassium hydroxide containing CO2 absorbents (e.g. BARALYME® ) are not recommended for use with sevoflurane.
-
ADVERSE REACTIONS
The most frequently reported adverse reactions during maintenance anesthesia were hypotension, followed by tachypnea, muscle tenseness, excitation, apnea, muscle fasciculations and emesis.
Infrequent adverse reactions include paddling, retching, salivation, cyanosis, premature ventricular contractions and excessive cardiopulmonary depression.
Transient elevations in liver function tests and white blood cell count may occur with sevoflurane, as with the use of other halogenated anesthetic agents.
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Dechra at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or online at www.fda.gov/reportanimalae.
-
PRECAUTIONS
Halogenated volatile anesthetics can react with desiccated carbon dioxide (CO2) absorbents to produce carbon monoxide (CO) that may result in elevated carboxyhemoglobin levels in some patients. To prevent this reaction, sevoflurane should not be passed through desiccated soda lime or barium hydroxide lime.
Replacement of Desiccated CO2 Absorbents:
When a clinician suspects that the CO2 absorbent may be desiccated, it should be replaced before administration of sevoflurane. The exothermic reaction that occurs with sevoflurane and CO2 absorbents is increased when the CO2 absorbent becomes desiccated, such as after an extended period of dry gas flow through the CO2 absorbent canisters. Extremely rare cases of spontaneous fire in the respiratory circuit of the anesthesia machine have been reported during sevoflurane use in conjunction with the use of a desiccated CO2 absorbent, specifically those containing potassium hydroxide (e.g. BARALYME®). Potassium hydroxide-containing CO2 absorbents are not recommended for use with sevoflurane. An unusually delayed rise in the inspired gas concentration (decreased delivery) of sevoflurane compared with the vaporizer setting may indicate excessive heating of the CO2 absorbent canister and chemical breakdown of sevoflurane. The color indicator of most CO2 absorbent may not change upon desiccation. Therefore, the lack of significant color change should not be taken as an assurance of adequate hydration. CO2 absorbents should be replaced routinely regardless of the state of the color indicator.
The use of some anesthetic regimens that include sevoflurane may result in bradycardia that is reversible with anticholinergics. Studies using sevoflurane anesthetic regimens that included atropine or glycopyrrolate as premedicants showed these anticholinergics to be compatible with sevoflurane in dogs.
During the induction and maintenance of anesthesia, increasing the concentration of sevoflurane produces dose dependent decreases in blood pressure and respiratory rate. Due to sevoflurane's low solubility in blood, these changes may occur more rapidly than with other volatile anesthetics. Excessive decreases in blood pressure or respiratory depression may be related to depth of anesthesia and may be corrected by decreasing the inspired concentration of sevoflurane. RESPIRATION MUST BE MONITORED CLOSELY IN THE DOG AND SUPPORTED WHEN NECESSARY WITH SUPPLEMENTAL OXYGEN AND/OR ASSISTED VENTILATION. The low solubility of sevoflurane also facilitates rapid elimination by the lungs.
The use of sevoflurane in humans increases both the intensity and duration of neuromuscular blockade induced by nondepolarizing muscle relaxants. The use of sevoflurane with nondepolarizing muscle relaxants has not been evaluated in dogs.
Compromised or debilitated dogs: Doses may need adjustment for geriatric or debilitated dogs. Because clinical experience in administering sevoflurane to dogs with renal, hepatic and cardiovascular insufficiency is limited, its safety in these dogs has not been established.
-
HUMAN SAFETY
Not for human use. Keep out of reach of children.
Operating rooms and animal recovery areas should be provided with adequate ventilation to prevent the accumulation of anesthetic vapors.
There is no specific work exposure limit established for sevoflurane. However, the National Institute for Occupational Safety and Health has recommended an 8 hour time-weighted average limit of 2 ppm for halogenated anesthetic agents in general.
Direct exposure to eyes may result in mild irritation. If eye exposure occurs, flush with plenty of water for 15 minutes. Seek medical attention if irritation persists.
Symptoms of human overexposure (inhalation) to sevoflurane vapors include respiratory depression, hypotension, bradycardia, shivering, nausea and headache. If these symptoms occur, remove the individual from the source of exposure and seek medical attention.
The safety data sheet contains more detailed occupational safety information. To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Dechra at (866) 933-2472.
-
CLINICAL PHARMACOLOGY
Sevoflurane is an inhalational anesthetic agent for induction and maintenance of general anesthesia. The Minimum Alveolar Concentration (MAC) of sevoflurane as determined in 18 dogs is 2.36%.2 MAC is defined as that alveolar concentration at which 50% of healthy patients fail to respond to noxious stimuli. Multiples of MAC are used as a guide for surgical levels of anesthesia, which are typically 1.3 to 1.5 times the MAC value.
Because of the low solubility of sevoflurane in blood (blood/gas partition coefficient at 37ºC = 0.63-0.69), a minimal amount of sevoflurane is required to be dissolved in the blood before the alveolar partial pressure is in equilibrium with the arterial partial pressure. During sevoflurane induction, there is a rapid increase in alveolar concentration toward the inspired concentration.
Sevoflurane produces only modest increases in cerebral blood flow and metabolic rate, and has little or no ability to potentiate seizures.3 Sevoflurane has a variable effect on heart rate, producing increases or decreases depending on experimental conditions.4, 5 Sevoflurane produces dose-dependent decreases in mean arterial pressure, cardiac output and myocardial contraction.6 Among inhalation anesthetics, sevoflurane has low arrhythmogenic potential.7
Sevoflurane is chemically stable. No discernible degradation occurs in the presence of strong acids or heat. Sevoflurane reacts through direct contact with CO2 absorbents (soda lime and barium hydroxide lime) producing pentafluoroisopropenyl fluoromethyl ether (PIFE, C4H2F6O), also known as Compound A, and trace amounts of pentafluoromethoxy isopropyl fluoromethyl ether (PMFE, C5H6F6O), also known as Compound B.
Compound A:
The production of degradants in the anesthesia circuit results from the extraction of the acidic proton in the presence of a strong base (potassium hydroxide and/or NaOH) forming an alkene (Compound A) from sevoflurane.
Compound A is produced when sevoflurane interacts with soda lime or barium hydroxide lime. Reaction with barium hydroxide lime results in a greater production of Compound A than does reaction with soda lime. Its concentration in a circle absorber system increases with increasing sevoflurane concentrations and with decreasing fresh gas flow rates. Sevoflurane degradation in soda lime has been shown to increase with temperature. Since the reaction of carbon dioxide with absorbents is exothermic, this temperature increase will be determined by the quantities of CO2 absorbed, which in turn will depend on fresh gas flow in the anesthetic circle system, metabolic status of the patient and ventilation. Although Compound A is a dose-dependent nephrotoxin in rats, the mechanism of this renal toxicity is unknown. Two spontaneously breathing dogs under sevoflurane anesthesia showed increases in concentrations of Compound A as the oxygen flow rate was decreased at hourly intervals, from 500 mL/min (36 and 18 ppm Compound A) to 250 mL/min in (43 and 31 ppm) to 50 mL/min (61 and 48 ppm).8
Fluoride ion metabolite:
Sevoflurane is metabolized to hexafluoroisopropanol (HFIP) with release of inorganic fluoride and CO2. Fluoride ion concentrations are influenced by the duration of anesthesia and the concentration of sevoflurane. Once formed, HFIP is rapidly conjugated with glucuronic acid and eliminated as a urinary metabolite. No other metabolic pathways for sevoflurane have been identified. In humans, the fluoride ion half-life was prolonged in patients with renal impairment, but human clinical trials contained no reports of toxicity associated with elevated fluoride ion levels. In a study in which 4 dogs were exposed to 4% sevoflurane for 3 hours, maximum serum fluoride concentrations of 17.0-27.0 mcmole/L were observed after 3 hours of anesthesia. Serum fluoride fell quickly after anesthesia ended, and had returned to baseline by 24 hours post-anesthesia.
In a safety study, eight healthy dogs were exposed to sevoflurane for 3 hours/day, 5 days/week for 2 weeks (total 30 hours exposure) at a flow rate of 500 mL/min in a semiclosed, rebreathing system with soda lime. Renal toxicity was not observed in the study evaluation of clinical signs, hematology, serum chemistry, urinalysis, or gross or microscopic pathology.
-
DRUG INTERACTIONS
In the clinical trial, sevoflurane was used safely in dogs that received frequently used veterinary products including steroids and heartworm and flea preventative products.
Intravenous Anesthetics: Sevoflurane administration is compatible with barbiturates, propofol and other commonly used intravenous anesthetics.
Benzodiazepines and Opioids: Benzodiazepines and opioids would be expected to decrease the MAC of sevoflurane in the same manner as other inhalational anesthetics. Sevoflurane is compatible with benzodiazepines and opioids as commonly used in surgical practice.
Phenothiazines and Alpha2-Agonists: Sevoflurane is compatible with phenothiazines and alpha2-agonists as commonly used in surgical practice. In a laboratory study, the use of the acepromazine/oxymorphone/thiopental/sevoflurane anesthetic regimen resulted in prolonged recoveries in eight (of 8) dogs compared to recoveries from sevoflurane alone.
-
CLINICAL EFFECTIVENESS
The effectiveness of sevoflurane was investigated in a clinical study involving 196 dogs. Thirty dogs were mask-induced with sevoflurane using anesthetic regimens that included various premedicants. During the clinical study, one hundred sixty-six dogs received sevoflurane maintenance anesthesia as part of several anesthetic regimens that used injectable induction agents and various premedicants.
The duration of anesthesia and the choice of anesthetic regimens were dependent upon the procedures that were performed. Duration of anesthesia ranged from 16 to 424 minutes among the individual dogs. Sevoflurane vaporizer concentrations during the first 30 minutes of maintenance anesthesia were similar among the various anesthetic regimens. The quality of maintenance anesthesia was considered good or excellent in 169 out of 196 dogs.
The table shows the average vaporizer concentrations and oxygen flow rates during the first 30 minutes for all sevoflurane maintenance anesthesia regimens:
Average Vaporizer Concentrations among Anesthetic Regimens Average Vaporizer Concentrations among Individual Dogs Average Oxygen Flow Rates among Anesthetic Regimens Average Oxygen Flow Rates among Individual Dogs 3.31-3.63% 1.6-5.1% 0.97-1.31 L/minute 0.5-3.0 L/minute During the clinical trial, when a barbiturate was used for induction, the times to extubation, sternal recumbency and standing recovery were longer for dogs that received anesthetic regimens containing two preanesthetics compared to regimens containing one preanesthetic. Recovery times were shorter when anesthetic regimens used sevoflurane or propofol for induction. The quality of recovery was considered good or excellent in 184 out of 196 dogs.
Anesthetic regimen drug dosages, physiological responses, and the quality of induction, maintenance and recovery were comparable between 10 sighthounds and other breeds evaluated in the study. During the clinical study there was no indication of prolonged recovery times in the sighthounds.
- HOW SUPPLIED
- STORAGE CONDITIONS
-
REFERENCES
- Plumb, D.C. ed., Veterinary Drug Handbook, Second Edition, University of Iowa Press, Ames, IA: p. 424 (1995).
- Kazama, T. and Ikeda, K., Comparison of MAC and the rate of rise of alveolar concentration of sevoflurane with halothane and isoflurane in the dog.
Anesthesiology. 68: 435-437 (1988). - Scheller, M.S., Nakakimura, K., Fleischer, J.E. and Zornow, M.H., Cerebral effects of sevoflurane in the dog: Comparison with isoflurane and enflurane.
Brit. J. Anesthesia 65: 388-392 (1990). - Frink, E.J., Morgan, S.E., Coetzee, A., Conzen, P.F. and Brown, B.R., Effects of sevoflurane, halothane, enflurane and isoflurane on hepatic blood flow and oxygenation in chronically instrumented greyhound dogs.
Anesthesiology 76: 85-90 (1992). - Kazama, T. and lkeda, K., The comparative cardiovascular effects of sevoflurane with halothane and isoflurane.
J. Anesthesiology 2: 63-8 (1988). - Bernard, J. M., Wouters, P.F., Doursout, M.F., Florence, B., Chelly, J.E. and Merin, R.G., Effects of sevoflurane on cardiac and coronary dynamics in chronically instrumented dogs.
Anesthesiology 72:659-662 (1990). - Hayaski, Y., Sumikawa, K., Tashiro, C., Yamatodani, A. and Yoshiya,I., Arrhythmogenic threshold of epinephrine during sevoflurane, enflurane and isoflurane anesthesia in dogs.
Anesthesiology 69:145-147 (1988). - Muir, W.W. and Gadawski, J., Cardiorespiratory effects of low-flow and closed circuit inhalation anesthesia, using sevoflurane delivered with an in-circuit vaporizer and concentrations of compound A.
Amer. J. Vet. Res. 59 (5): 603-608 (1998).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 250 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
SEVOSPIRE
sevoflurane liquidProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-092 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sevoflurane (UNII: 38LVP0K73A) (Sevoflurane - UNII:38LVP0K73A) Sevoflurane 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-092-25 1 in 1 CARTON 1 250 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200467 11/01/2024 Labeler - Dechra Veterinary Products LLC (362142734)


