Label: PAVBLU- aflibercept-ayyh injection, solution
- NDC Code(s): 55513-056-01, 55513-065-01
- Packager: Amgen, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PAVBLU safely and effectively. See full prescribing information for PAVBLU. PAVBLU™ (aflibercept-ayyh) injection, for intravitreal ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPAVBLU is indicated for the treatment of: 1.1 Neovascular (Wet) Age-Related Macular Degeneration (AMD) 1.2 Macular Edema Following Retinal Vein Occlusion (RVO) 1.3 Diabetic Macular ...
-
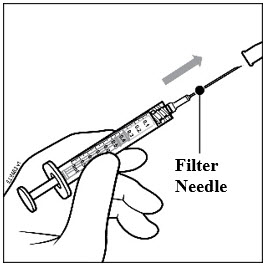
2 DOSAGE AND ADMINISTRATION2.1 Important Injection Instructions - For ophthalmic intravitreal injection. PAVBLU must only be administered by a qualified physician. Prefilled Syringe: A 30-gauge × ½-inch sterile injection ...
-
3 DOSAGE FORMS AND STRENGTHSPAVBLU is a clear to opalescent and colorless to slightly yellow solution available as: Injection: 2 mg (0.05 mL of 40 mg/mL solution) in a single-dose prefilled plastic syringe - Injection: 2 mg ...
-
4 CONTRAINDICATIONS4.1 Ocular or Periocular Infections - PAVBLU is contraindicated in patients with ocular or periocular infections. 4.2 Active Intraocular Inflammation - PAVBLU is contraindicated in patients ...
-
5 WARNINGS AND PRECAUTIONS5.1 Endophthalmitis, Retinal Detachments, and Retinal Vasculitis with or without Occlusion - Intravitreal injections, including those with aflibercept products, have been associated with ...
-
6. ADVERSE REACTIONSThe following potentially serious adverse reactions are described elsewhere in the labeling: Hypersensitivity [see Contraindications (4.3)] Endophthalmitis, retinal detachments, and Retinal ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Adequate and well-controlled studies with aflibercept have not been conducted in pregnant women. Aflibercept produced adverse embryofetal effects in rabbits ...
-
10 OVERDOSAGEOverdosing with increased injection volume may increase intraocular pressure. Therefore, in case of overdosage, intraocular pressure should be monitored and if deemed necessary by the treating ...
-
11 DESCRIPTIONAflibercept-ayyh is a recombinant fusion protein consisting of portions of human VEGF receptors 1 and 2 extracellular domains fused to the Fc portion of human IgG1 formulated as an iso-osmotic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Vascular endothelial growth factor-A (VEGF-A) and placental growth factor (PlGF) are members of the VEGF family of angiogenic factors that can act as mitogenic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted on the mutagenic or carcinogenic potential of aflibercept products. Effects on male and female fertility ...
-
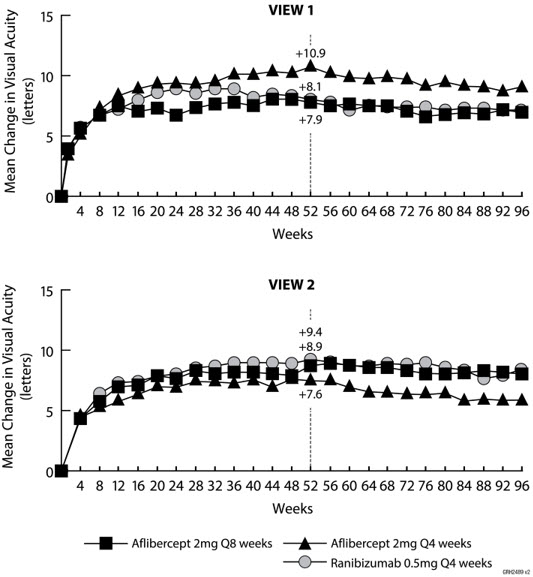
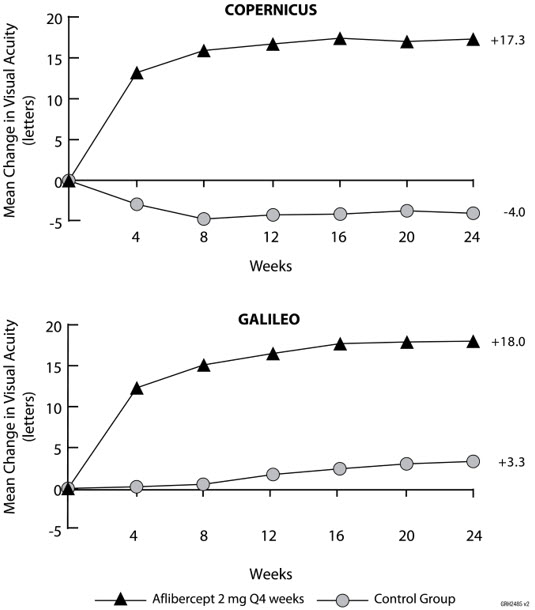
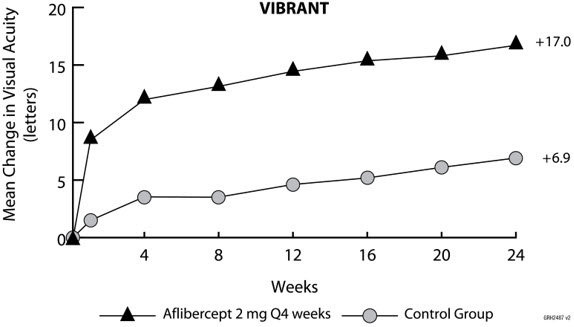
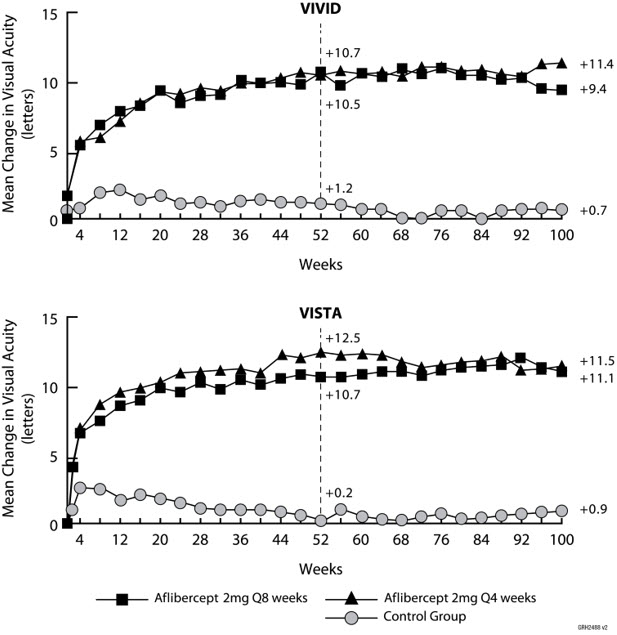
14 CLINICAL STUDIES14.1 Neovascular (Wet) Age-Related Macular Degeneration (AMD) The safety and efficacy of aflibercept were assessed in two randomized, multi-center, double-masked, active-controlled studies in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Each prefilled syringe or vial is for single eye use only. Discard unused portion. PAVBLU injection is a clear to opalescent and colorless to slightly yellow solution supplied ...
-
17 PATIENT COUNSELING INFORMATIONIn the days following PAVBLU administration, patients are at risk of developing endophthalmitis, retinal detachment, or retinal vasculitis with or without occlusion. If the eye becomes red ...
-
SPL UNCLASSIFIED SECTIONPAVBLU™ (aflibercept-ayyh) Manufactured by: Amgen, Inc. One Amgen Center Drive - Thousand Oaks, CA 91320-1799 - U.S. License Number 1080 - AMGEN® and PAVBLU™ (aflibercept-ayyh) are trademarks owned ...
-
PRINCIPAL DISPLAY PANEL - 0.05 mL Syringe Carton2 mg / 0.05 mL - PAVBLU™ (aflibercept-ayyh) Injection - NDC 55513-056-96 - Not for Sale - 2 mg (0.05 mL of 40 mg/mL solution) Carton contents: one blister pack containing one sterile, single-dose ...
-
PRINCIPAL DISPLAY PANEL - 0.05 mL Vial Carton2 mg / 0.05 mL - NDC 55513-065-01 - PAVBLU™ (aflibercept-ayyh) Injection - 2 mg (0.05 mL of - 40 mg/mL solution) For Intravitreal Injection - One Single-Dose Vial - Sterile Solution - Contains no ...
-
INGREDIENTS AND APPEARANCEProduct Information