Label: RYTELO- imetelstat sodium injection, powder, lyophilized, for solution
- NDC Code(s): 82959-111-01, 82959-112-01
- Packager: Geron Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RYTELO safely and effectively. See full prescribing information for RYTELO. RYTELO (imetelstat) for injection, for intravenous ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGERYTELO is indicated for the treatment of adult patients with low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia requiring 4 or more red blood cell units ...
-
2. DOSAGE AND ADMINISTRATION2.1. Recommended Dosage - The recommended dosage of RYTELO is 7.1 mg/kg administered as an intravenous infusion over 2 hours every 4 weeks. Discontinue RYTELO if a patient does not experience a ...
-
3. DOSAGE FORMS AND STRENGTHSFor injection: 47 mg of imetelstat supplied as a white to off-white or slightly yellow lyophilized powder in a single-dose vial for reconstitution. For injection: 188 mg of imetelstat supplied as ...
-
4. CONTRAINDICATIONSNone.
-
5. WARNINGS AND PRECAUTIONS5.1. Thrombocytopenia - RYTELO can cause thrombocytopenia based on laboratory values. In the clinical trial, new or worsening Grade 3 or 4 decreased platelets occurred in 65% of patients with MDS ...
-
6. ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Thrombocytopenia [see Warnings and Precautions (5.1)] Neutropenia [see Warnings and Precautions ...
-
8. USE IN SPECIFIC POPULATIONS8.1. Pregnancy - Risk Summary - Based on findings in animal studies, RYTELO can cause embryo-fetal harm when administered to a pregnant woman. There are no available data on RYTELO use in ...
-
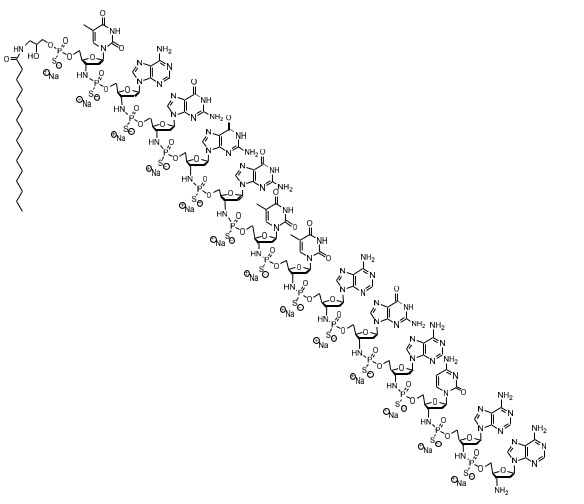
11. DESCRIPTIONRYTELO for injection contains imetelstat, an oligonucleotide telomerase inhibitor for intravenous use. Imetelstat sodium is a white to off-white or slightly yellow, amorphous, solid powder. It is ...
-
12. CLINICAL PHARMACOLOGY12.1. Mechanism of Action - Imetelstat is an oligonucleotide human telomerase inhibitor that binds to the template region of the RNA component of human telomerase (hTR), inhibits telomerase ...
-
13. NONCLINICAL TOXICOLOGY13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted with imetelstat. In vitro, imetelstat was not mutagenic in the bacterial mutagenicity ...
-
14. CLINICAL STUDIES14.1. Myelodysplastic Syndromes (MDS) The efficacy of RYTELO was evaluated in a randomized, double-blind, placebo-controlled, multicenter trial (IMerge; NCT02598661) in 178 patients enrolled ...
-
16. HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - RYTELO (imetelstat) for injection is a preservative free, white to off-white or slightly yellow, lyophilized powder available as: Carton ContentsNDC - One RYTELO 47 mg ...
-
17. PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Discuss the following with patients prior to and during treatment with RYTELO. Thrombocytopenia - Advise ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Geron Corporation - 919 E. Hillsdale Blvd., Suite 250 - Foster City, CA 94404 - Manufactured by (188 mg vials): Catalent Indiana, LLC - 1300 S Patterson Drive - Bloomington, IN ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Issued: 6/2024 MEDICATION GUIDE - RYTELO™ (ri-TEL-o) (imetelstat) for injection, for intravenous ...
-
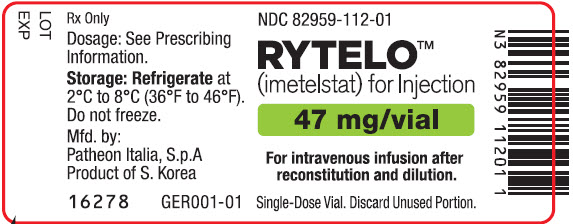
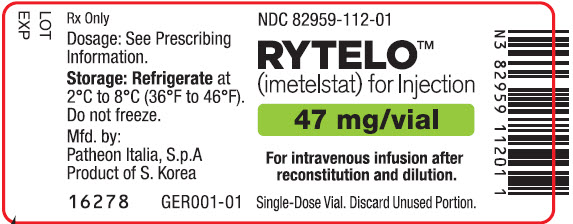
PRINCIPAL DISPLAY PANEL - 47 mg Vial LabelNDC 82959-112-01 - RYTELO™ (imetelstat) for Injection - 47 mg/vial - For intravenous infusion after - reconstitution and dilution. Single-Dose Vial. Discard Unused Portion.
-
PRINCIPAL DISPLAY PANEL - 47 mg Vial CartonNDC 82959-112-01 - Rx Only - RYTELO™ (imetelstat) for Injection - 47 mg/vial - For intravenous infusion after - reconstitution and dilution. Dispense the enclosed Medication - Guide to each ...
-
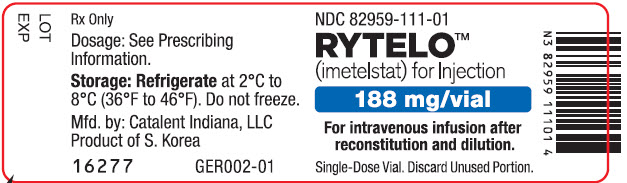
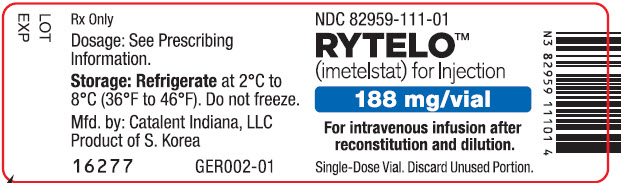
PRINCIPAL DISPLAY PANEL - 188 mg Vial LabelNDC 82959-111-01 - RYTELO™ (imetelstat) for Injection - 188 mg/vial - For intravenous infusion after - reconstitution and dilution. Single-Dose Vial. Discard Unused Portion.
-
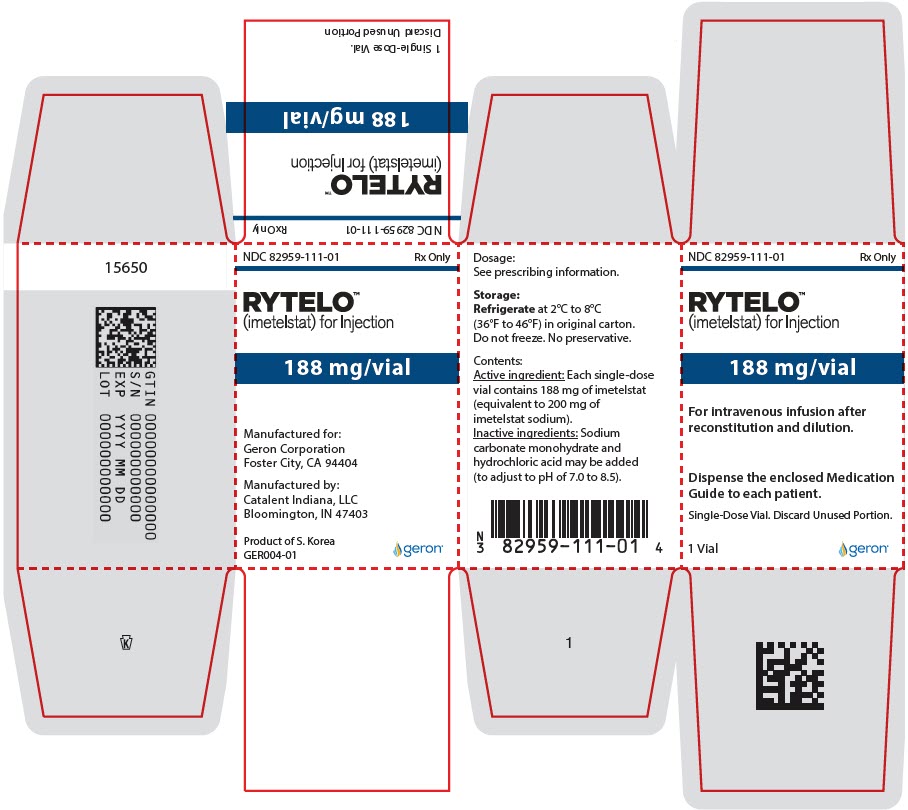
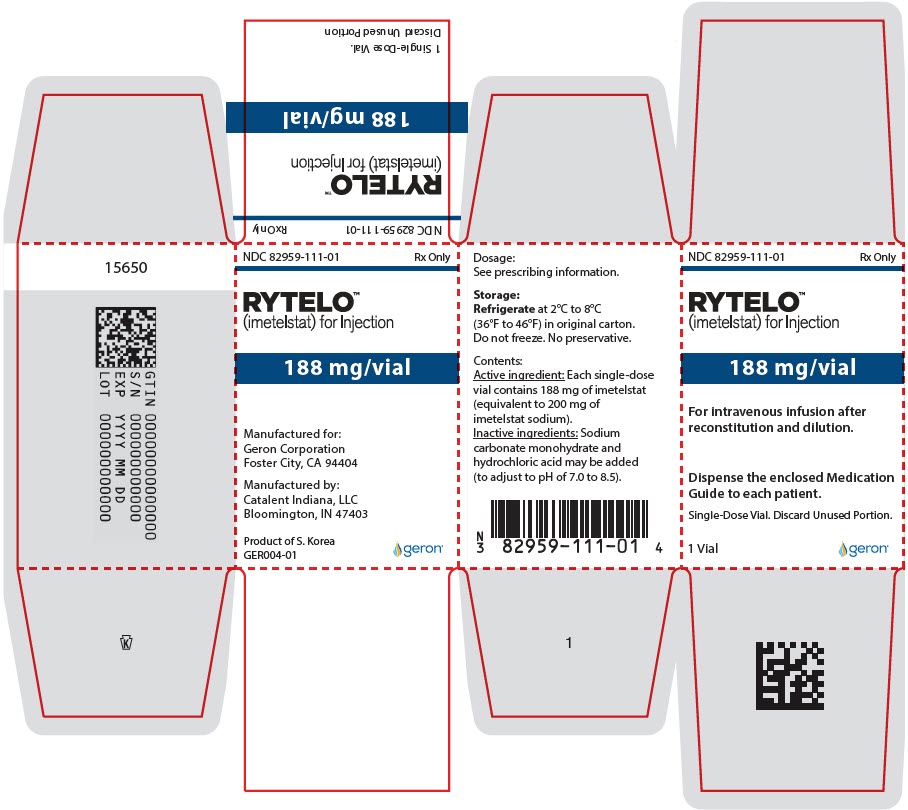
PRINCIPAL DISPLAY PANEL - 188 mg Vial CartonNDC 82959-111-01 - Rx Only - RYTELO™ (imetelstat) for Injection - 188 mg/vial - For intravenous infusion after - reconstitution and dilution. Dispense the enclosed Medication - Guide to each ...
-
INGREDIENTS AND APPEARANCEProduct Information