Label: DICYCLOMINE HYDROCHLORIDE capsule
- NDC Code(s): 24979-201-01, 24979-201-03
- Packager: Upsher-Smith Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 6, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DICYCLOMINE HYDROCHLOLRIDE CAPSULES safely and effectively. See full prescribing information for DICYCLOMINE HYDROCHLOLRIDE CAPSULES ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDicyclomine hydrochloride capsules are indicated for the treatment of patients with functional bowel/irritable bowel syndrome.
-
2 DOSAGE AND ADMINISTRATIONDosage must be adjusted to individual patient needs. 2.1 Oral Dosage and Administration in Adults - The recommended initial dose is 20 mg four times a day. After one week treatment with the ...
-
3 DOSAGE FORMS AND STRENGTHSDicyclomine Hydrochloride Capsules USP, 10 mg: white capsules with "TWi T201" ink and white body with"10 mg" ink, and the capsule contains white to off white powder blend.
-
4 CONTRAINDICATIONSDicyclomine hydrochloride is contraindicated in infants less than 6 months of age [see Use in Specific Populations (8.4)], nursing mothers [see Use in Specific Populations (8.3)], and in patients ...
-
5 WARNINGS AND PRECAUTIONS5.2 Cardiovascular Conditions - Dicyclomine hydrochloride capsules need to be used with caution in conditions characterized by tachyarrhythmia such as thyrotoxicosis, congestive heart failure and ...
-
6 ADVERSE REACTIONSThe pattern of adverse effects seen with dicyclomine is mostly related to its pharmacological actions at muscarinic receptors [see Clinical Pharmacology (12)]. They are a consequence of the ...
-
7 DRUG INTERACTIONS7.1 Antiglaucoma Agents - Anticholinergics antagonize the effects of antiglaucoma agents. Anticholinergic drugs in the presence of increased intraocular pressure may be hazardous when taken ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category B - Adequate and well-controlled studies have not been conducted with dicyclomine hydrochloride in pregnant women at the recommended doses of 80 to 160 mg/day ...
-
10 OVERDOSAGEIn case of an overdose, patients should contact a physician, poison control center (1-800-222-1222), or emergency room. The signs and symptoms of overdosage include: headache; nausea; vomiting ...
-
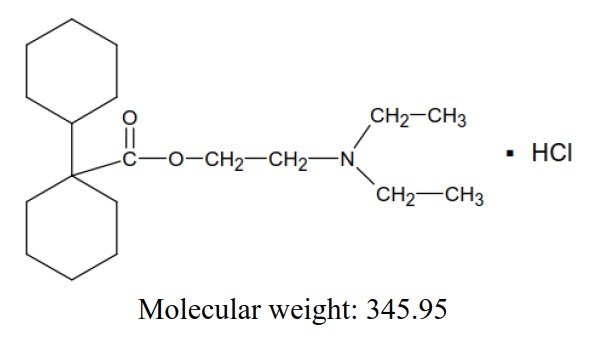
11 DESCRIPTIONDicyclomine hydrochloride capsules, USP, are antispasmodic and anticholinergic (antimuscarinic) agent available in the following dosage forms: Dicyclomine Hydrochloride Capsules, USP, 10 mg for ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Dicyclomine relieves smooth muscle spasm of the gastrointestinal tract. Animal studies indicate that this action is achieved via a dual mechanism: a specific ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been conducted to evaluate the carcinogenic potential of dicyclomine. In studies in rats at doses of ...
-
14 CLINICAL STUDIESIn controlled clinical trials involving over 100 patients who received drug, 82% of patients treated for functional bowel/irritable bowel syndrome with dicyclomine hydrochloride capsules at ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDicyclomine Hydrochloride Capsules, USP, 10 mg - 10 mg white capsules with "TWi T201" ink and white body with"10 mg" ink, and the capsule contains white to off white powder blend, supplied in ...
-
17 PATIENT COUNSELING INFORMATION17.2 Use in Infants - Inform parents and caregivers not to administer dicyclomine hydrochloride in infants less than 6 months of age [see Use in Specific Populations (8.4)]. 17.3 Use in ...
-
PRINCIPAL DISPLAY PANEL — Dicyclomine HCl Capsules, USP Bottle LabelNDC 24979-201-01 - Dicyclomine Hydrochloride Capsules, USP - 10 mg - Rx Only - 100 Capsules - TWi
-
PRINCIPAL DISPLAY PANEL — Dicyclomine HCl Capsules, USP Bottle LabelNDC 24979-201-01 - Dicyclomine Hydrochloride Capsules, USP - 10 mg - Rx Only - 1000 Capsules - TWi
-
INGREDIENTS AND APPEARANCEProduct Information