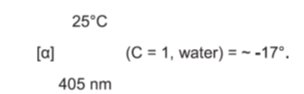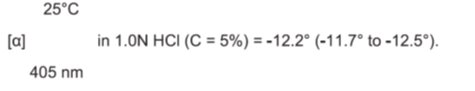Label: DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE solution/ drops
- NDC Code(s): 24208-486-05, 24208-486-10
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Dorzolamide hydrochloride and timolol maleate ophthalmic solution is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension ...
-
2 DOSAGE AND ADMINISTRATION The dose is one drop of dorzolamide hydrochloride and timolol maleate ophthalmic solution in the affected eye(s) two times daily. If more than one topical ophthalmic drug is being used, the drugs ...
-
3 DOSAGE FORMS AND STRENGTHS Dorzolamide hydrochloride and timolol maleate ophthalmic solution, USP containing dorzolamide 20 mg/mL (2%) equivalent to 22.26 mg/mL of dorzolamide hydrochloride and timolol 5 mg/mL (0.5% ...
-
4 CONTRAINDICATIONS 4.1 Asthma, COPD - Dorzolamide hydrochloride and timolol maleate ophthalmic solution is contraindicated in patients with bronchial asthma, a history of bronchial asthma, or severe chronic ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Potentiation of Respiratory Reactions Including Asthma - Dorzolamide hydrochloride and timolol maleate ophthalmic solution contains timolol maleate, a beta-adrenergic blocking agent; and ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS 7.1 Oral Carbonic Anhydrase Inhibitors - There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Teratogenic Effects. Developmental toxicity studies with dorzolamide hydrochloride in rabbits at oral doses of ≥2.5 mg/kg/day (37 times the recommended human ophthalmic dose ...
-
10 OVERDOSAGE Symptoms consistent with systemic administration of beta-blockers or carbonic anhydrase inhibitors may occur, including electrolyte imbalance, development of an acidotic state, dizziness ...
-
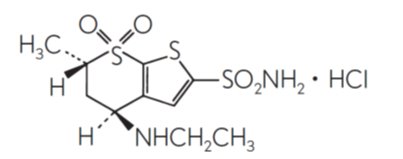
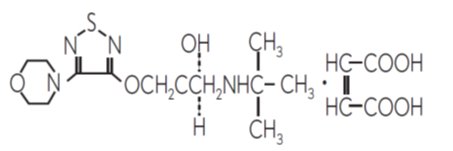
11 DESCRIPTION Dorzolamide hydrochloride and timolol maleate ophthalmic solution, USP is the combination of a topical carbonic anhydrase inhibitor and a topical beta-adrenergic receptor blocking agent ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Dorzolamide hydrochloride and timolol maleate ophthalmic solution is comprised of two components: dorzolamide hydrochloride and timolol maleate. Each of these two ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a two-year study of dorzolamide hydrochloride administered orally to male and female Sprague-Dawley rats, urinary bladder ...
-
14 CLINICAL STUDIES Clinical studies of 3 to 15 months duration were conducted to compare the IOP-lowering effect over the course of the day of dorzolamide hydrochloride and timolol maleate ophthalmic solution twice ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Dorzolamide hydrochloride and timolol maleate ophthalmic solution USP, 2% / 0.5% is supplied in a white low-density polyethylene (LDPE) bottle with a white LDPE dropper tip and a blue ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-Approved patient labeling (Patient Information and Instructions for Use). Potential for Exacerbation of Asthma and COPD - Dorzolamide hydrochloride and timolol ...
-
PATIENT INFORMATION Dorzolamide Hydrochloride and Timolol Maleate Ophthalmic Solution, USP - (dor-ZOE-la-mide HYE-droe-KLOR-ide and TIM-oh-lol MAL-ee-ate) for topical ophthalmic use - What is dorzolamide ...
-
INSTRUCTIONS FOR USE Dorzolamide Hydrochloride and Timolol Maleate Ophthalmic Solution, USP - (dor-ZOE-la-mide HYE-droe-KLOR-ide and TIM-oh-lol MAL-ee-ate) for topical ophthalmic use - Read this Instructions for Use ...
-
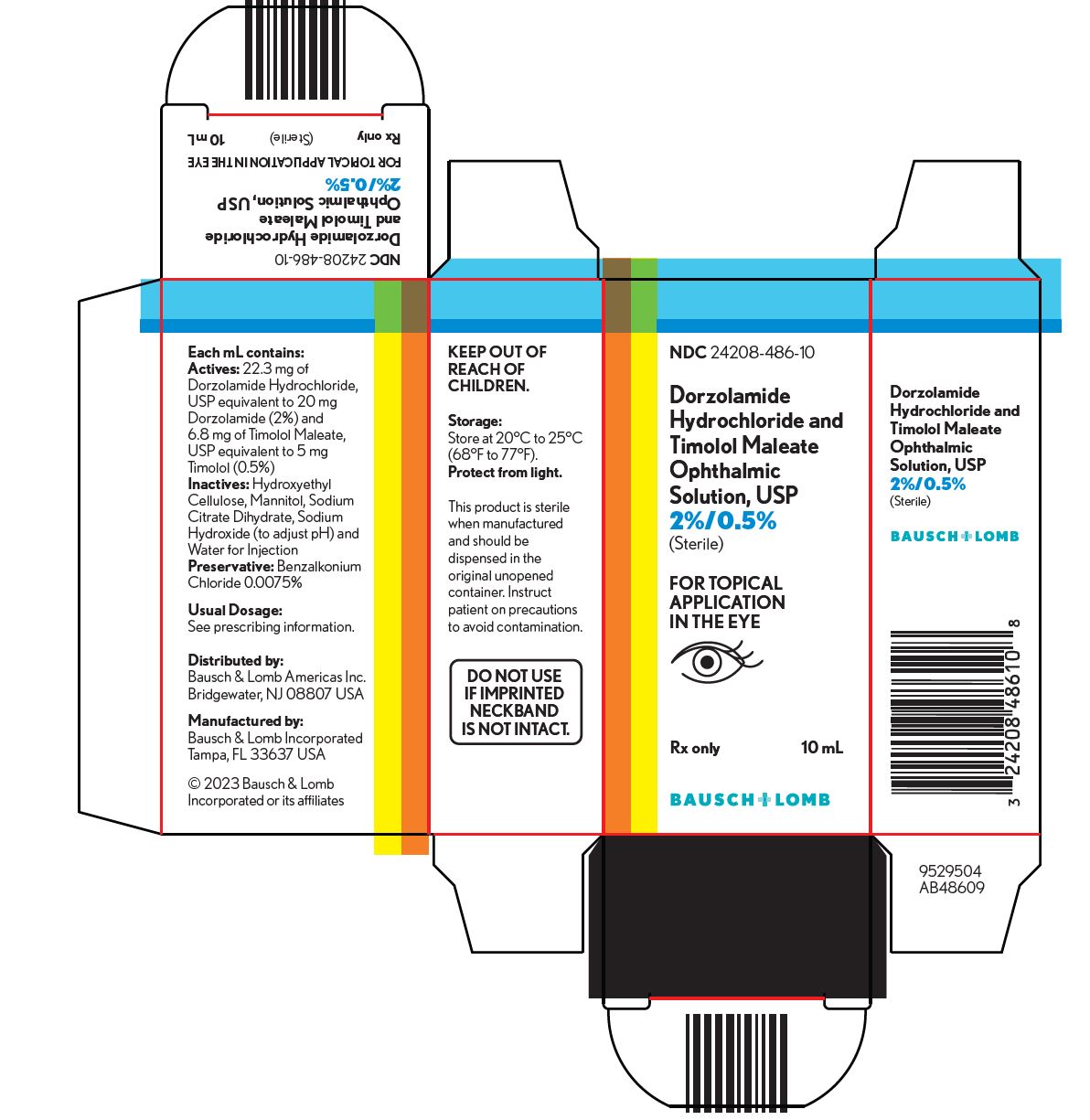
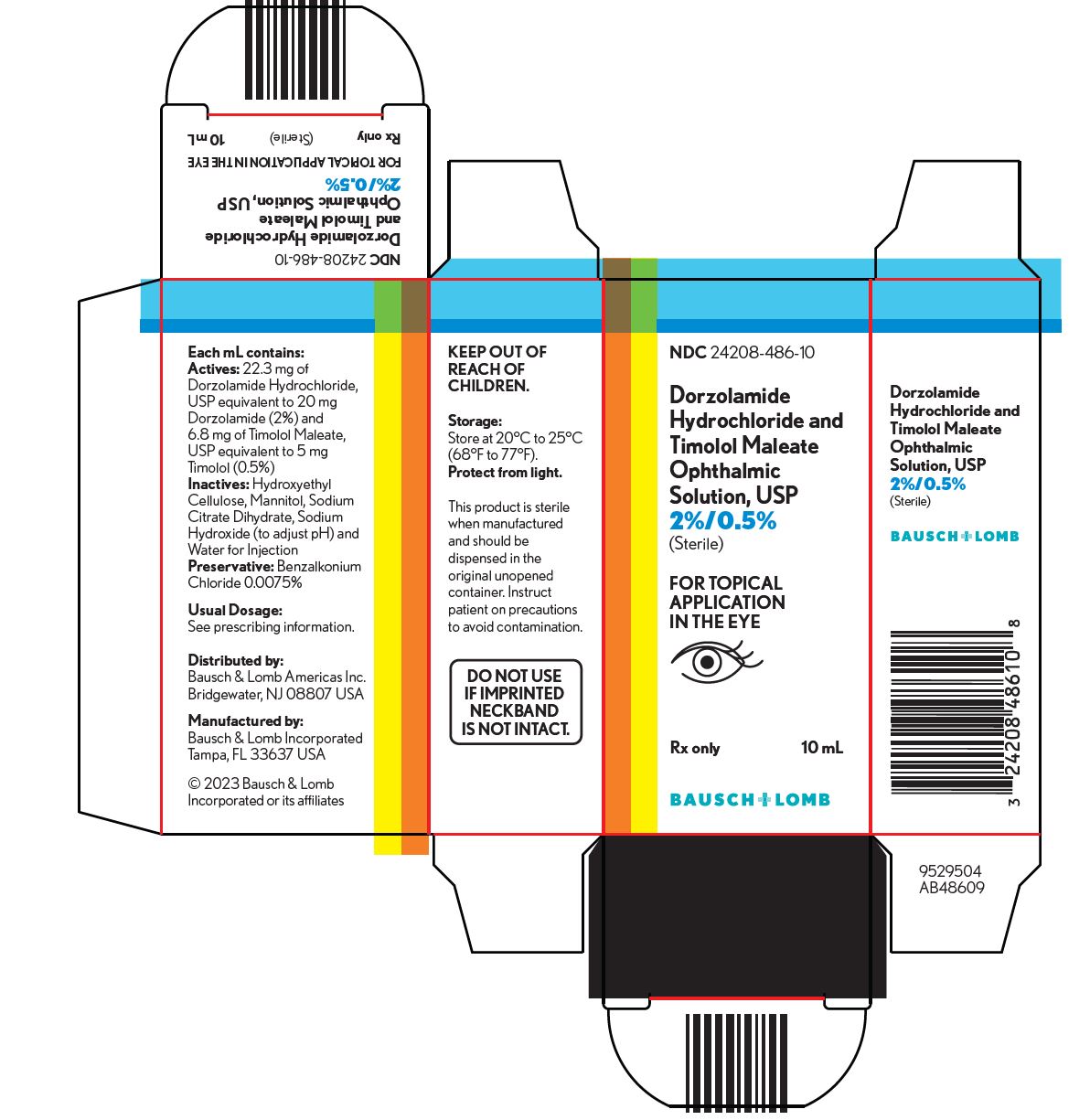
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 24208-486-10 - Dorzolamide - Hydrochloride and - Timolol Maleate - Ophthalmic - Solution, USP - 2%/0.5% (Sterile) FOR TOPICAL - APPLICATION - IN THE EYE - Rx only 10 ...
-
INGREDIENTS AND APPEARANCEProduct Information