Label: LATANOPROST solution/ drops
- NDC Code(s): 24208-463-25
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LATANOPROST OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for LATANOPROST OPHTHALMIC ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Latanoprost ophthalmic solution is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
-
2 DOSAGE AND ADMINISTRATION The recommended dosage is one drop in the affected eye(s) once daily in the evening. If one dose is missed, treatment should continue with the next dose as normal. The dosage of latanoprost ...
-
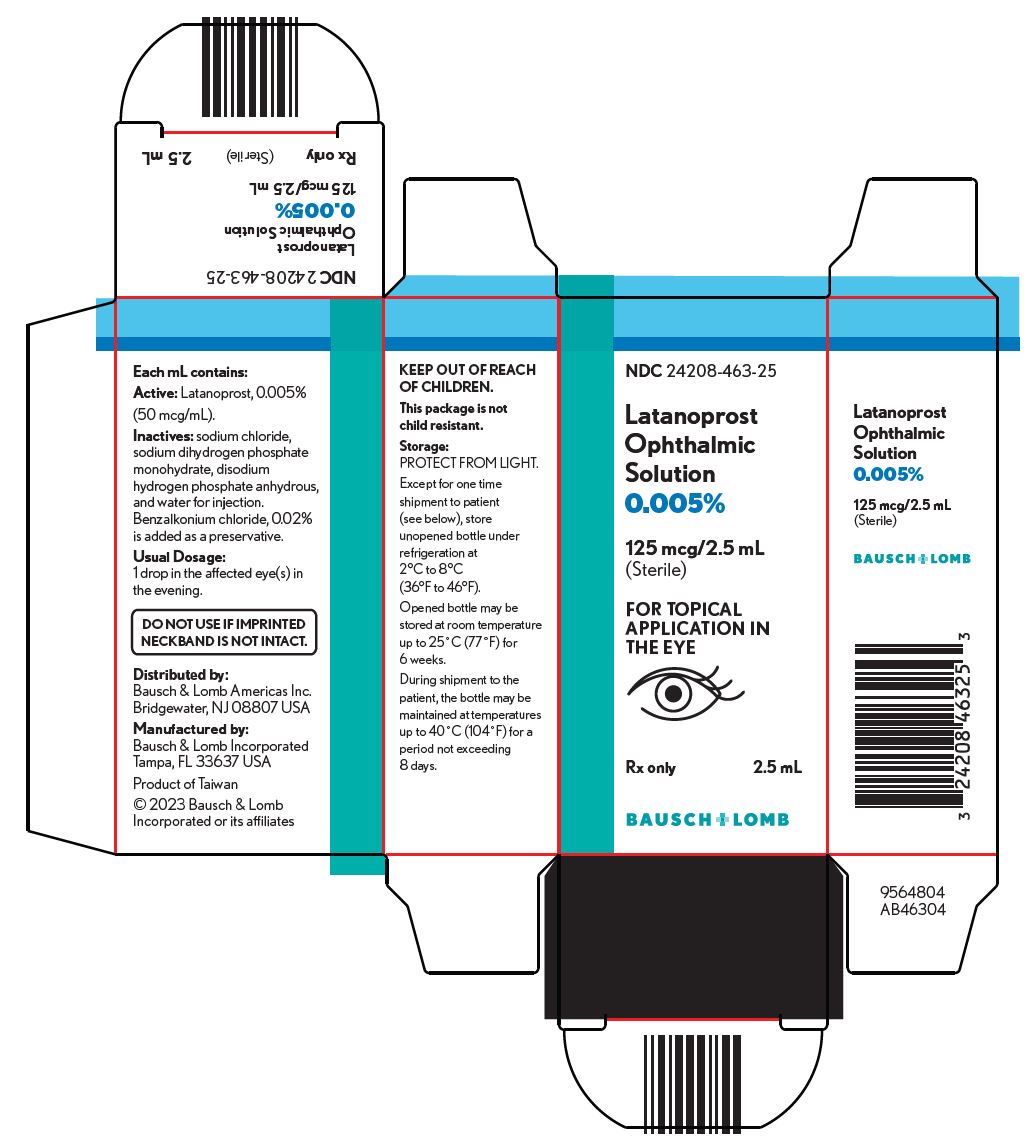
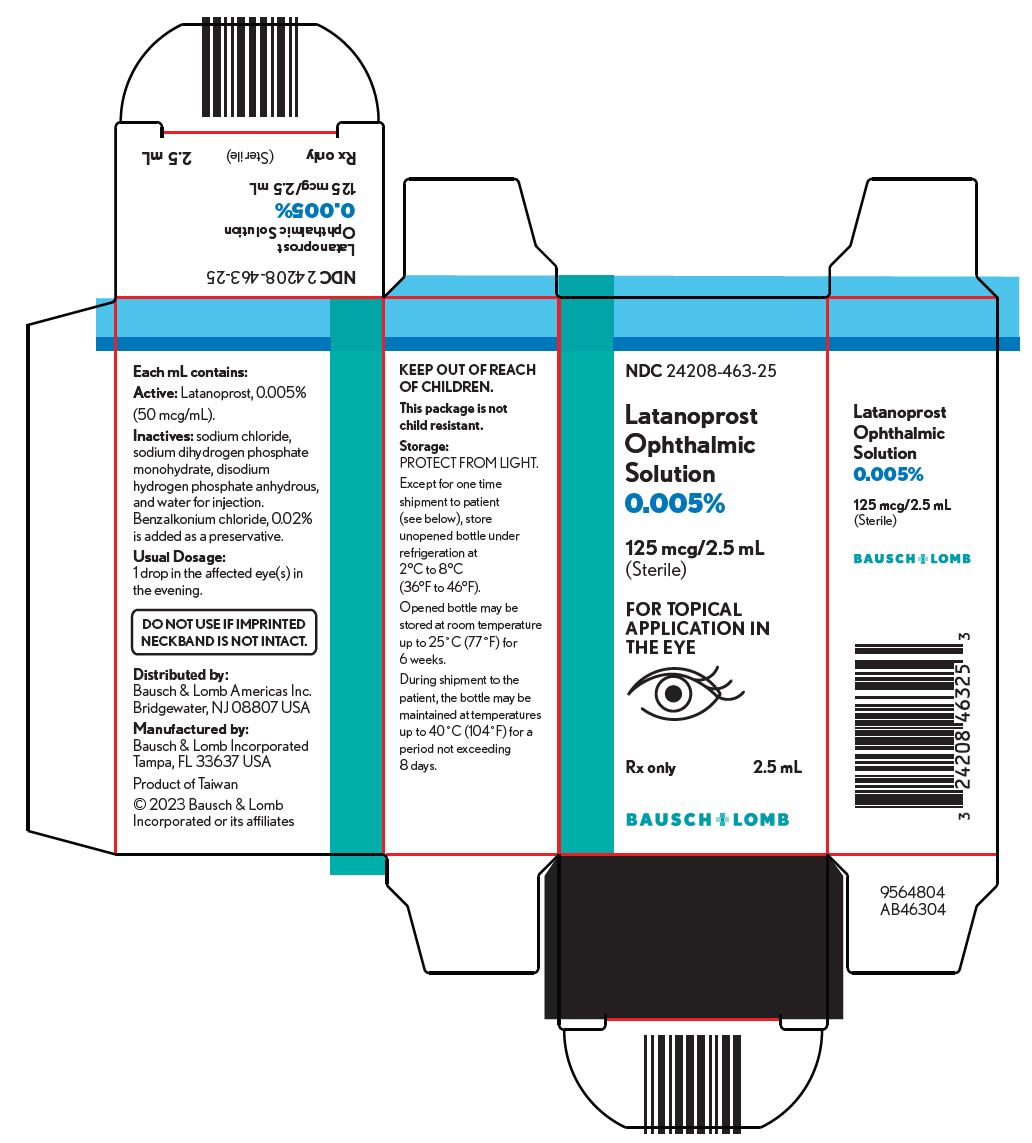
3 DOSAGE FORMS AND STRENGTHS Ophthalmic solution containing latanoprost 50 mcg/mL (0.005%).
-
4 CONTRAINDICATIONS Known hypersensitivity to latanoprost, benzalkonium chloride, or any other ingredients in this product.
-
5 WARNINGS AND PRECAUTIONS 5.1 Pigmentation - Latanoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris ...
-
6 ADVERSE REACTIONS The following adverse reactions were reported in postmarketing experience and are discussed in greater detail in other sections of the label: • Iris pigmentation changes [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of latanoprost ophthalmic solution administration in pregnant women to inform drug-associated risks. In animal ...
-
10 OVERDOSAGE IV infusion of up to 3 mcg/kg of latanoprost in healthy volunteers produced mean plasma concentrations 200 times higher than during clinical treatment with latanoprost ophthalmic solution and no ...
-
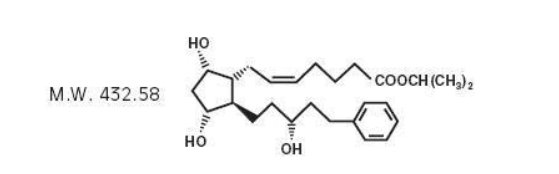
11 DESCRIPTION Latanoprost is a prostaglandin F2α analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Latanoprost is a prostaglandin F2α analogue that is believed to reduce the IOP by increasing the outflow of aqueous humor. Studies in animals and man suggest that the ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Latanoprost was not carcinogenic in either mice or rats when administered by oral gavage at doses of up to 170 ...
-
14 CLINICAL STUDIES 14.1 Elevated Baseline IOP - Patients with mean baseline IOP of 24 - 25 mmHg who were treated for 6 months in multi-center, randomized, controlled trials demonstrated 6 - 8 mmHg reductions in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Latanoprost ophthalmic solution is a clear, isotonic, buffered, preserved colorless solution of latanoprost 50 mcg/mL (0.005%). It is supplied as a 2.5 mL solution in a 4 mL clear low density ...
-
17 PATIENT COUNSELING INFORMATION Potential for Pigmentation - Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Inform patients about the possibility of eyelid skin ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 24208-463-25 - Latanoprost - Ophthalmic - Solution - 0.005% 125 mcg/2.5 mL - (Sterile) FOR TOPICAL - APPLICATION IN - THE EYE - Rx only 2.5 mL - 9564804 - BAUSCH ...
-
INGREDIENTS AND APPEARANCEProduct Information