Label: DICLOFENAC SODIUM solution/ drops
- NDC Code(s): 24208-457-05, 24208-457-25
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
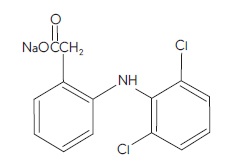
DESCRIPTIONDiclofenac sodium ophthalmic solution, 0.1% is a sterile, topical, nonsteroidal, anti-inflammatory product for ophthalmic use. Diclofenac sodium is designated chemically as ...
-
CLINICAL PHARMACOLOGYPharmacodynamics - Diclofenac sodium is one of a series of phenylacetic acids that has demonstrated anti-inflammatory and analgesic properties in pharmacological studies. It is thought to inhibit ...
-
INDICATIONS AND USAGEDiclofenac sodium ophthalmic solution is indicated for the treatment of postoperative inflammation in patients who have undergone cataract extraction and for the temporary relief of pain and ...
-
CONTRAINDICATIONSDiclofenac sodium ophthalmic solution is contraindicated in patients who are hypersensitive to any component of the medication.
-
WARNINGSThe refractive stability of patients undergoing corneal refractive procedures and treated with diclofenac sodium has not been established. Patients should be monitored for a year following use in ...
-
PRECAUTIONSGeneral - All topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs ...
-
ADVERSE REACTIONSOcular - Transient burning and stinging were reported in approximately 15% of patients across studies with the use of diclofenac sodium ophthalmic solution. In cataract surgery studies, keratitis ...
-
OVERDOSAGEOverdosage will not ordinarily cause acute problems. If diclofenac sodium ophthalmic solution is accidentally ingested, fluids should be taken to dilute the medication.
-
DOSAGE AND ADMINISTRATIONCataract Surgery - One drop of diclofenac sodium ophthalmic solution should be applied to the affected eye, 4 times daily beginning 24 hours after cataract surgery and continuing throughout the ...
-
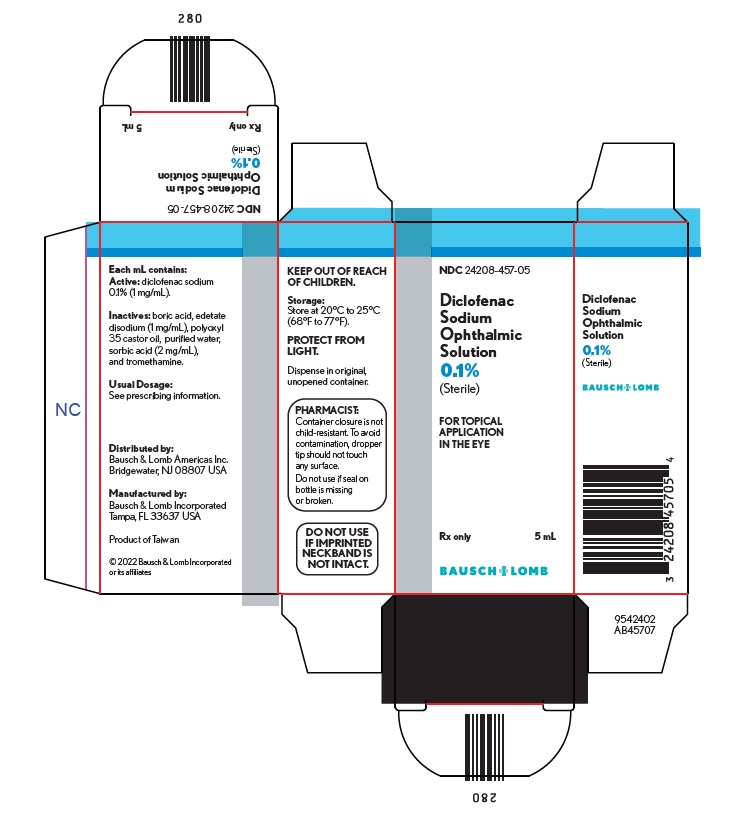
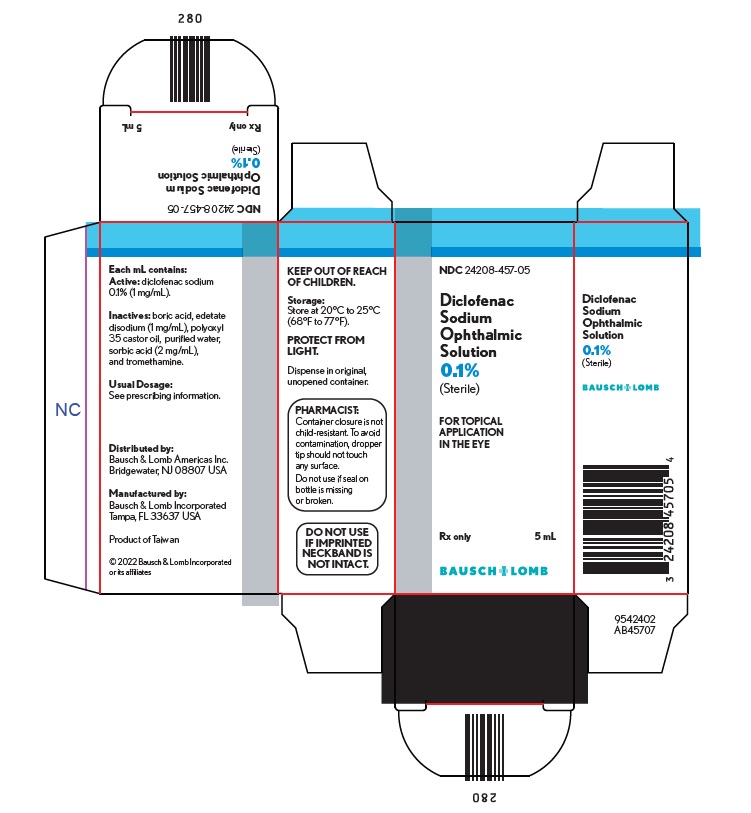
HOW SUPPLIEDDiclofenac sodium ophthalmic solution, 0.1% (1 mg/mL) sterile solution is supplied in a low-density polyethylene (LDPE) white bottle with a LDPE Dropper Tip and a polypropylene gray cap. The 2.5 ...
-
Medication GuideDistributed by: Bausch & Lomb Americas Inc. Bridgewater, NJ 08807 USA - Manufactured by: Bausch & Lomb Incorporated - Tampa, FL 33637 USA - © 2022 Bausch & Lomb ...
-
Package/Label Display Panel – 5 mL cartonNDC24208-457-05 - Diclofenac - Sodium - Ophthalmic - Solution - 0.1% (Sterile) FOR TOPICAL - APPLICATION - IN THE EYE - Rx only - 5 mL - BAUSCH + LOMB - 9542403 - AB45707
-
INGREDIENTS AND APPEARANCEProduct Information