Label: ISOSORBIDE MONONITRATE tablet, extended release
- NDC Code(s): 23155-178-01, 23155-178-05, 23155-178-09, 23155-519-01, view more
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 2, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
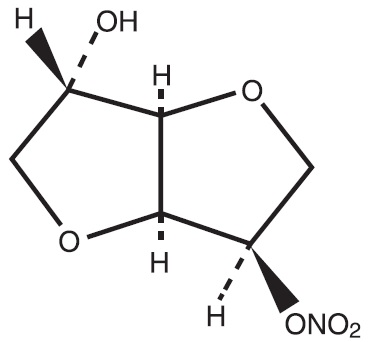
Isosorbide mononitrate (ISMN), an organic nitrate and the major biologically active metabolite of isosorbide dinitrate (ISDN), is a vasodilator with effects on both arteries and veins. Each ...
-
CLINICAL PHARMACOLOGY
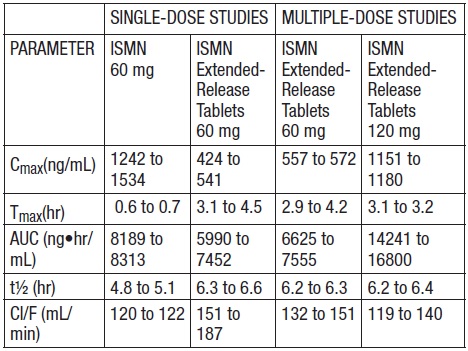
Mechanism of Action - The Isosorbide Mononitrate Extended-Release Tablet, USP is an oral extended-release formulation of ISMN, the major active metabolite of isosorbide dinitrate; most of the ...
-
INDICATIONS AND USAGE
Isosorbide Mononitrate Extended-Release Tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of oral isosorbide mononitrate is not ...
-
CONTRAINDICATIONS
Isosorbide Mononitrate Extended-Release Tablets are contraindicated in patients who have shown hypersensitivity or idiosyncratic reactions to other nitrates or nitrites.
-
WARNINGS
Amplification of the vasodilatory effects of isosorbide mononitrate by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied ...
-
PRECAUTIONS
General - Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide mononitrate. This drug should, therefore, be used with caution in patients who may ...
-
ADVERSE REACTIONS
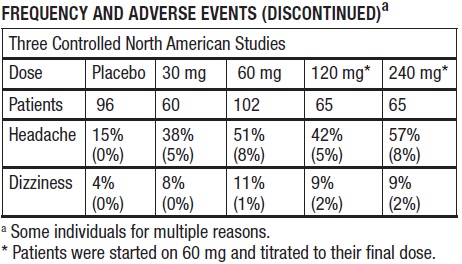
The table below shows the frequencies of the adverse events that occurred in >5% of the subjects in three placebo-controlled North American studies, in which patients in the active treatment arm ...
-
OVERDOSAGE
Hemodynamic Effects - The ill effects of isosorbide mononitrate overdose are generally the result of isosorbide mononitrate's capacity to induce vasodilatation, venous pooling, reduced cardiac ...
-
DOSAGE AND ADMINISTRATION
The recommended starting dose of Isosorbide Mononitrate Extended-Release Tablets is 30 mg (given as a single 30 mg tablet or as 1/2 of a 60 mg tablet) or 60 mg (given as a single tablet) once ...
-
HOW SUPPLIED
Isosorbide Mononitrate Extended-Release Tablets, USP 30 mg are White, biconvex oval shaped tablets, scored and embossed "30" on one side. Bottles of 30 NDC 23155-519-03 - Bottles of 100 NDC ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 30 mg
Isosorbide Mononitrate Extended-Release Tablets, USP - 30 mg - 100 Tablets - Rx Only
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 60 mg
Isosorbide Mononitrate Extended-Release Tablets, USP - 60 mg - 100 Tablets - Rx Only
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 120 mg
Isosorbide Mononitrate Extended-Release Tablets, USP - 120 mg - 100 Tablets - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information