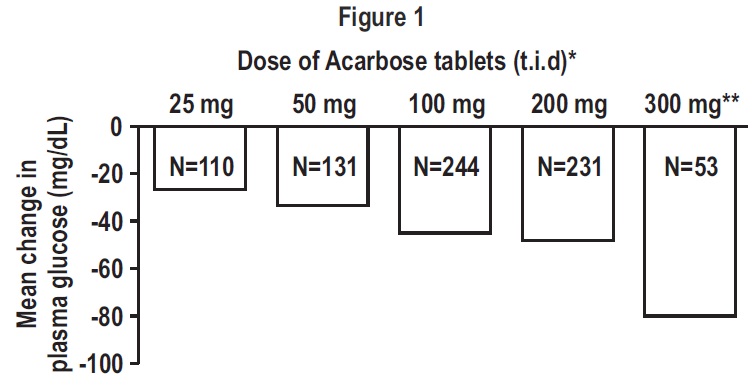
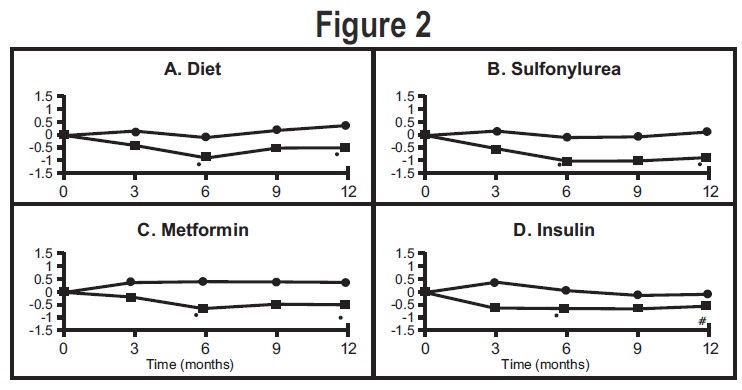
General
-
Macrovascular Outcomes
-
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with acarbose tablets or any other anti-diabetic ...
General
Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with acarbose tablets or any other anti-diabetic drug.
Hypoglycemia
Because of its mechanism of action, acarbose tablets when administered alone should not cause hypoglycemia in the fasted or postprandial state. Sulfonylurea agents or insulin may cause hypoglycemia. Because acarbose given in combination with a sulfonylurea or insulin will cause a further lowering of blood glucose, it may increase the potential for hypoglycemia. Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, and no increased incidence of hypoglycemia was observed in patients when acarbose was added to metformin therapy. Oral glucose (dextrose), whose absorption is not inhibited by acarbose tablets, should be used instead of sucrose (cane sugar) in the treatment of mild to moderate hypoglycemia. Sucrose, whose hydrolysis to glucose and fructose is inhibited by acarbose tablets, is unsuitable for the rapid correction of hypoglycemia. Severe hypoglycemia may require the use of either intravenous glucose infusion or glucagon injection.
Elevated Serum Transaminase Levels
In long-term studies (up to 12 months, and including acarbose tablets doses up to 300 mg t.i.d.) conducted in the United States, treatment emergent elevations of serum transaminases (AST and/or ALT) above the upper limit of normal (ULN), greater than 1.8 times the ULN, and greater than 3 times the ULN occurred in 14%, 6%, and 3%, respectively, of acarbose-treated patients as compared to 7%, 2%, and 1%, respectively, of placebo-treated patients. Although these differences between treatments were statistically significant, these elevations were asymptomatic, reversible, more common in females, and, in general, were not associated with other evidence of liver dysfunction. In addition, these serum transaminase elevations appeared to be dose related. In US studies including acarbose doses up to the maximum approved dose of 100 mg t.i.d., treatment-emergent elevations of AST and/or ALT at any level of severity were similar between acarbose-treated patients and placebo-treated patients (p ≥ 0.496).
In approximately 3 million patient-years of international postmarketing experience with acarbose tablets, 62 cases of serum transaminase elevations > 500 IU/L (29 of which were associated with jaundice) have been reported. Forty-one of these 62 patients received treatment with 100 mg t.i.d. or greater and 33 of 45 patients for whom weight was reported weighed < 60 kg. In the 59 cases where follow-up was recorded, hepatic abnormalities improved or resolved upon discontinuation of acarbose tablets in 55 and were unchanged in two. Cases of fulminant hepatitis with fatal outcome have been reported; the relationship to acarbose is unclear.
Loss of Control of Blood Glucose
When diabetic patients are exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of control of blood glucose may occur. At such times, temporary insulin therapy may be necessary.
Information for Patients
Patients should be told to take acarbose tablets orally three times a day at the start (with the first bite) of each main meal. It is important that patients continue to adhere to dietary instructions, a regular exercise program, and regular testing of urine and/or blood glucose.
Acarbose tablets itself do not cause hypoglycemia even when administered to patients in the fasted state. Sulfonylurea drugs and insulin, however, can lower blood sugar levels enough to cause symptoms or sometimes life-threatening hypoglycemia. Because acarbose tablets given in combination with a sulfonylurea or insulin will cause a further lowering of blood sugar, it may increase the hypoglycemic potential of these agents. Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, and no increased incidence of hypoglycemia was observed in patients when acarbose tablets were added to metformin therapy. The risk of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be well understood by patients and responsible family members. Because acarbose prevents the breakdown of table sugar, patients should have a readily available source of glucose (dextrose, D-glucose) to treat symptoms of low blood sugar when taking acarbose tablets in combination with a sulfonylurea or insulin.
If side effects occur with acarbose tablets, they usually develop during the first few weeks of therapy. They are most commonly mild-to-moderate gastrointestinal effects, such as flatulence, diarrhea, or abdominal discomfort, and generally diminish in frequency and intensity with time.
Laboratory Tests
Therapeutic response to acarbose tablets should be monitored by periodic blood glucose tests. Measurement of glycosylated hemoglobin levels is recommended for the monitoring of long-term glycemic control.
Acarbose tablets, particularly at doses in excess of 50 mg t.i.d., may give rise to elevations of serum transaminases and, in rare instances, hyperbilirubinemia. It is recommended that serum transaminase levels be checked every 3 months during the first year of treatment with acarbose tablets and periodically thereafter. If elevated transaminases are observed, a reduction in dosage or withdrawal of therapy may be indicated, particularly if the elevations persist.
Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking acarbose. Use alternative methods to monitor for glycemic control.
Renal Impairment
Plasma concentrations of acarbose in renally impaired volunteers were proportionally increased relative to the degree of renal dysfunction. Long-term clinical trials in diabetic patients with significant renal dysfunction (serum creatinine > 2.0 mg/dL) have not been conducted. Therefore, treatment of these patients with acarbose tablets is not recommended.
Drug Interactions
Certain drugs tend to produce hyperglycemia and may lead to loss of blood glucose control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel-blocking drugs, and isoniazid. When such drugs are administered to a patient receiving acarbose tablets, the patient should be closely observed for loss of blood glucose control. When such drugs are withdrawn from patients receiving acarbose tablets in combination with sulfonylureas or insulin, patients should be observed closely for any evidence of hypoglycemia.
Patients Receiving Sulfonylureas or Insulin: Sulfonylurea agents or insulin may cause hypoglycemia. Acarbose tablets given in combination with a sulfonylurea or insulin may cause a further lowering of blood glucose and may increase the potential for hypoglycemia.
If hypoglycemia occurs, appropriate adjustments in the dosage of these agents should be made. Very rarely, individual cases of hypoglycemic shock have been reported in patients receiving acarbose tablets therapy in combination with sulfonylureas and/or insulin.
Intestinal adsorbents (for example, charcoal) and digestive enzyme preparations containing carbohydrate-splitting enzymes (for example, amylase, pancreatin) may reduce the effect of acarbose tablets and should not be taken concomitantly.
Acarbose tablets has been shown to change the bioavailability of digoxin when they are co administered, which may require digoxin dose adjustment. (See CLINICAL PHARMACOLOGY, Drug-Drug Interactions).
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Eight carcinogenicity studies were conducted with acarbose. Six studies were performed in rats (two strains, Sprague-Dawley and Wistar) and two studies were performed in hamsters.
In the first rat study, Sprague-Dawley rats received acarbose in feed at high doses (up to approximately 500 mg/kg body weight) for 104 weeks. Acarbose treatment resulted in a significant increase in the incidence of renal tumors (adenomas and adenocarcinomas) and benign Leydig cell tumors. This study was repeated with a similar outcome. Further studies were performed to separate direct carcinogenic effects of acarbose from indirect effects resulting from the carbohydrate malnutrition induced by the large doses of acarbose employed in the studies. In one study using Sprague-Dawley rats, acarbose was mixed with feed but carbohydrate deprivation was prevented by the addition of glucose to the diet. In a 26-month study of Sprague-Dawley rats, acarbose was administered by daily postprandial gavage so as to avoid the pharmacologic effects of the drug. In both of these studies, the increased incidence of renal tumors found in the original studies did not occur. Acarbose was also given in food and by postprandial gavage in two separate studies in Wistar rats. No increased incidence of renal tumors was found in either of these Wistar rat studies. In two feeding studies of hamsters, with and without glucose supplementation, there was also no evidence of carcinogenicity.
Acarbose did not induce any DNA damage in vitro in the CHO chromosomal aberration assay, bacterial mutagenesis (Ames) assay, or a DNA binding assay. In vivo, no DNA damage was detected in the dominant lethal test in male mice, or the mouse micronucleus test. Fertility studies conducted in rats after oral administration produced no untoward effect on fertility or on the overall capability to reproduce.
Pregnancy
Teratogenic Effects: Pregnancy Category B.
The safety of acarbose tablets in pregnant women has not been established. Reproduction studies have been performed in rats at doses up to 480 mg/kg (corresponding to 9 times the exposure in humans, based on drug blood levels) and have revealed no evidence of impaired fertility or harm to the fetus due to acarbose. In rabbits, reduced maternal body weight gain, probably the result of the pharmacodynamic activity of high doses of acarbose in the intestines may have been responsible for a slight increase in the number of embryonic losses. However, rabbits given 160 mg/kg acarbose (corresponding to 10 times the dose in man, based on body surface area) showed no evidence of embryotoxicity and there was no evidence of teratogenicity at a dose 32 times the dose in man (based on body surface area). There are, however, no adequate and well-controlled studies of acarbose tablets in pregnant women. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed. Because current information strongly suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital anomalies as well as increased neonatal morbidity and mortality, most experts recommend that insulin be used during pregnancy to maintain blood glucose levels as close to normal as possible.
Nursing Mothers
A small amount of radioactivity has been found in the milk of lactating rats after administration of radiolabeled acarbose. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, acarbose tablets should not be administered to a nursing woman.
Pediatric Use
Safety and effectiveness of acarbose tablets in pediatric patients have not been established.
Geriatric Use
Of the total number of subjects in clinical studies of acarbose tablets in the United States, 27 % were 65 and over, while 4 % were 75 and over. No overall differences in safety and effectiveness were observed between these subjects and younger subjects.
The mean steady-state area under the curve (AUC) and maximum concentrations of acarbose were approximately 1.5 times higher in elderly compared to young volunteers; however, these differences were not statistically significant.
Close