Label: NARATRIPTAN tablet
- NDC Code(s): 23155-054-03, 23155-054-05, 23155-054-19, 23155-055-03, view more
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NARATRIPTAN TABLETS safely and effectively. See full prescribing information for NARATRIPTAN TABLETS. NARATRIPTAN tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENaratriptan tablets are indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use: Use only if a clear diagnosis of migraine has been established ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended dose of naratriptan tablets is 1 mg or 2.5 mg. If the migraine returns or if the patient has only partial response, the dose may be repeated once after 4 ...
-
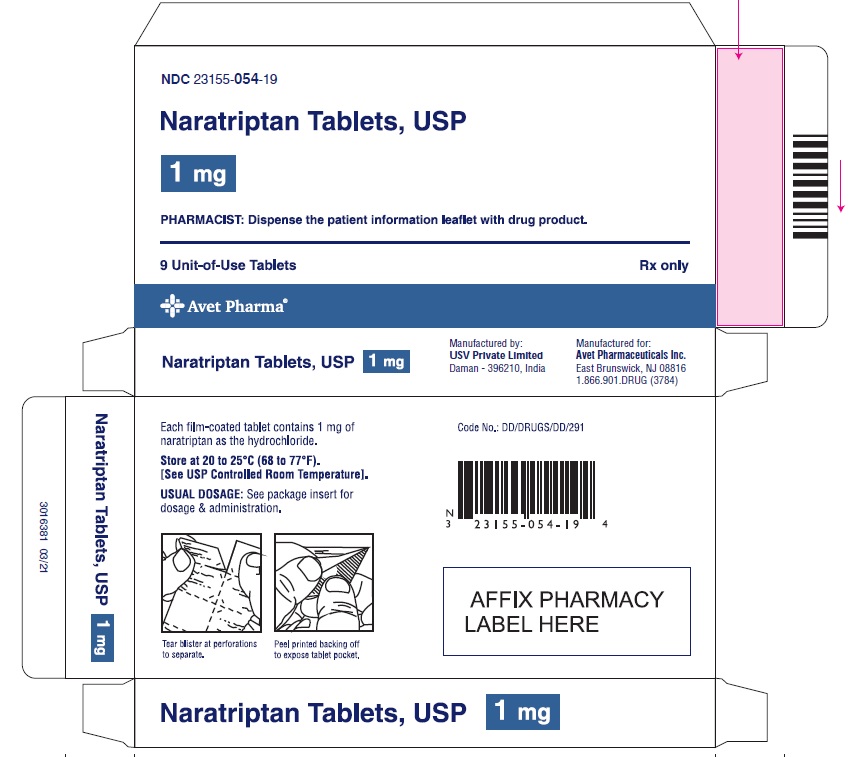
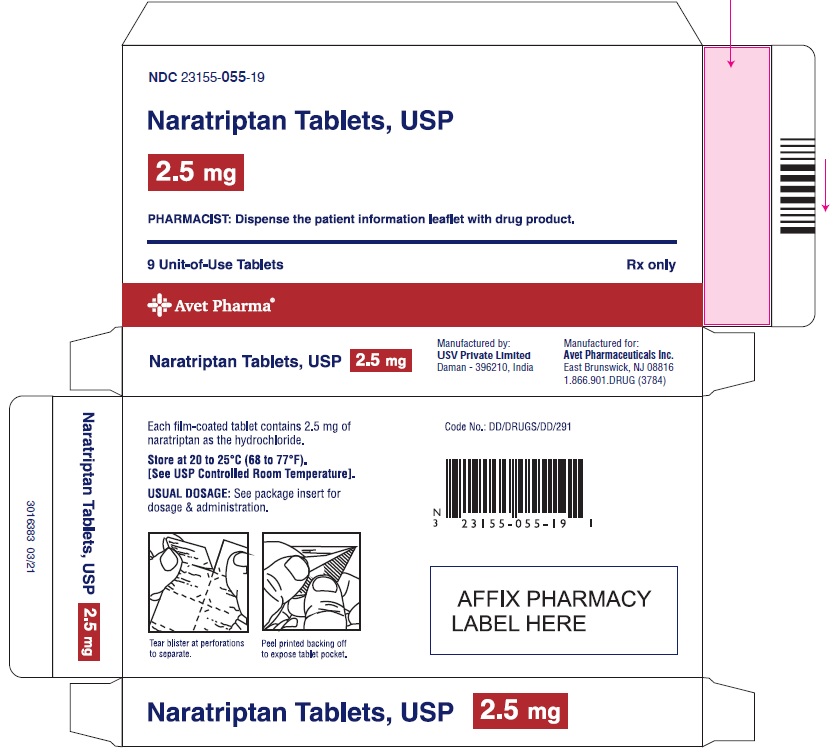
3 DOSAGE FORMS AND STRENGTHS1-mg yellow, round, biconvex film-coated tablets, debossed with 'I53' 2.5-mg white, 'D' shaped, biconvex film-coated tablets, debossed with 'I54'
-
4 CONTRAINDICATIONSNaratriptan tablets are contraindicated in patients with: Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina - Naratriptan is contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the prescribing information: Myocardial ischemia, myocardial infarction, and Prinzmetal's angina [see ...
-
7 DRUG INTERACTIONS7.1 Ergot-Containing Drugs - Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with use of naratriptan in pregnant women. Data from a prospective pregnancy exposure registry and ...
-
10 OVERDOSAGEAdverse reactions observed after overdoses of up to 25 mg included increases in blood pressure resulting in lightheadedness, neck tension, tiredness, and loss of coordination. Also, ischemic ECG ...
-
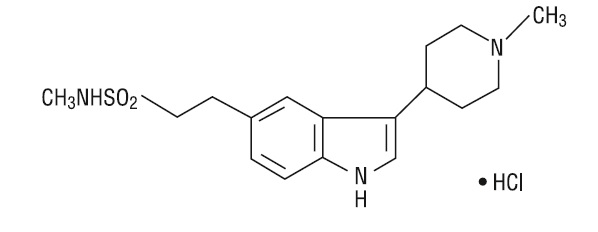
11 DESCRIPTIONNaratriptan tablets, USP contains naratriptan hydrochloride, a selective 5-HT1B/1D receptor agonist. Naratriptan hydrochloride is chemically designated as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Naratriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Migraines are likely due to local cranial vasodilatation and/or to the release of sensory ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: In carcinogenicity studies, mice and rats were given naratriptan by oral gavage for 104 weeks. There was no evidence ...
-
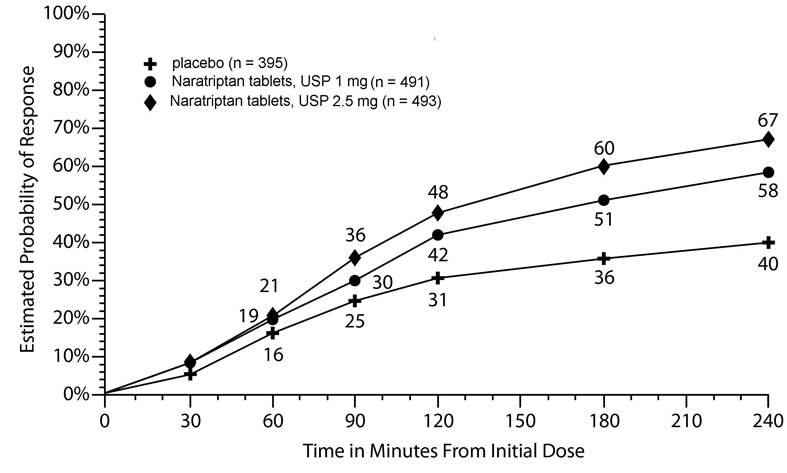
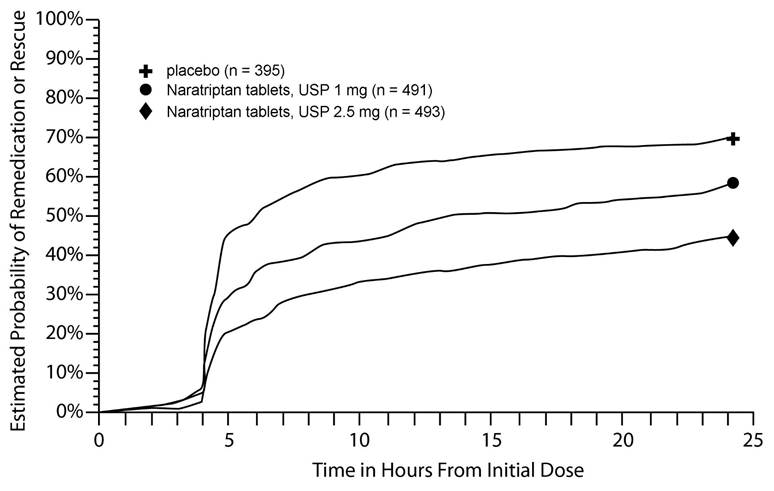
14 CLINICAL STUDIESThe efficacy of naratriptan in the acute treatment of migraine headaches was evaluated in 3 randomized, double-blind, placebo-controlled trials in adult patients (Trials 1, 2, 3). These trials ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNaratriptan tablets, USP containing 1 mg and 2.5 mg of naratriptan (base) as the hydrochloride. Naratriptan tablets, USP 1 mg, are yellow, round, biconvex film-coated tablets, debossed with 'I53 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Risk of Myocardial Ischemia and/or Infarction, Prinzmetal's Angina, Other Vasospasm-Related Events ...
-
Patient InformationDispense with Patient Information available at: www.avetpharma.com/product - Patient Information - NARATRIPTAN TABLETS, USP - (NAR-a-TRIP-tan) Read this Patient Information before you start ...
-
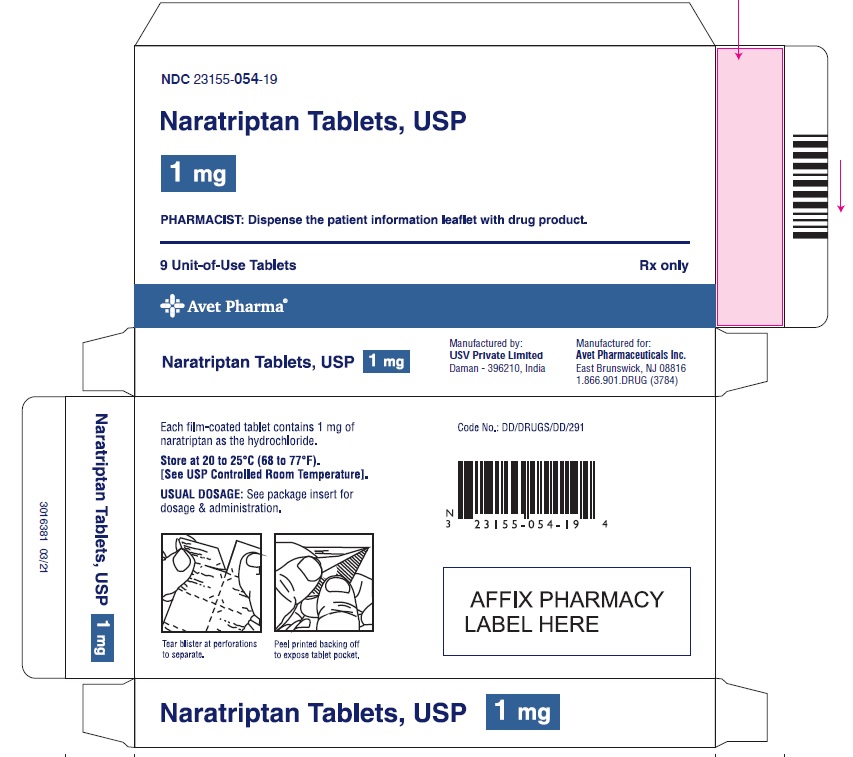
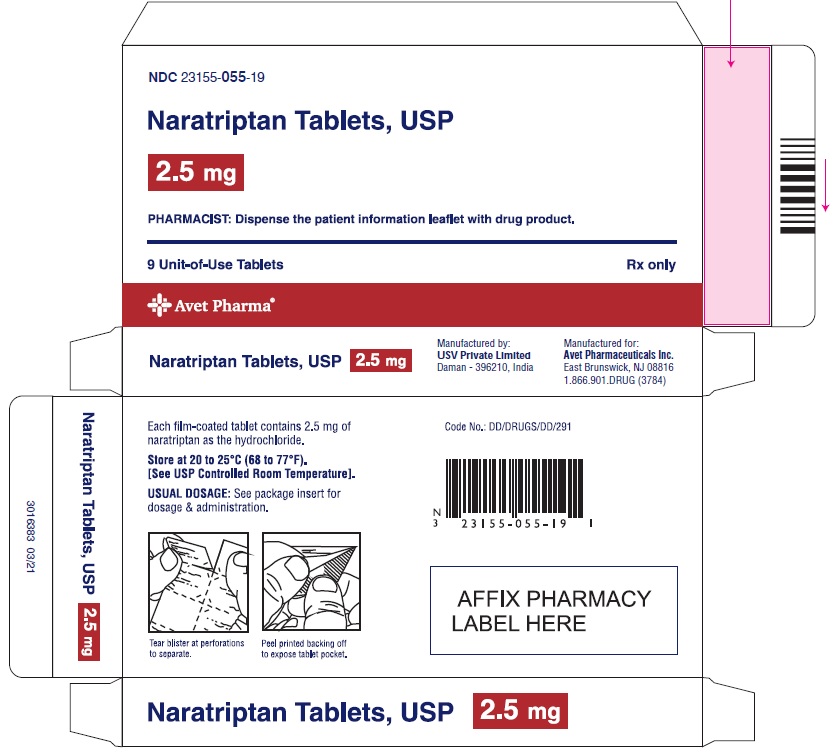
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNaratriptan Tablets, USP 1 mg, 9 Unit-of-Use Tablets - Naratriptan Tablets, USP 2.5 mg, 9 Unit-of-Use Tablets
-
INGREDIENTS AND APPEARANCEProduct Information