Label: HYDRALAZINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 23155-001-01, 23155-001-10, 23155-002-01, 23155-002-10, view more
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
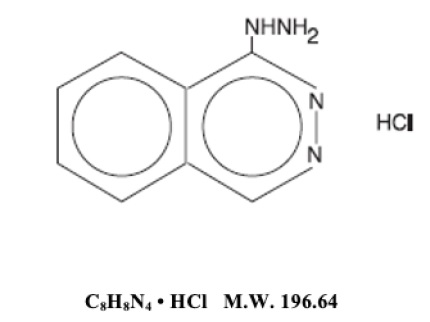
DESCRIPTIONHydrALAZINE hydrochloride, USP, is an antihypertensive, for oral administration. Its chemical name is 1-hydrazinophthalazine monohydrochloride, and its structural formula is: HydrALAZINE ...
-
CLINICAL PHARMACOLOGYAlthough the precise mechanism of action of hydrALAZINE is not fully understood, the major effects are on the cardiovascular system. HydrALAZINE apparently lowers blood pressure by exerting a ...
-
INDICATIONS AND USAGEEssential hypertension, alone or as an adjunct.
-
CONTRAINDICATIONSHypersensitivity to hydrALAZINE; coronary artery disease; mitral valvular rheumatic heart disease.
-
WARNINGSIn a few patients hydrALAZINE may produce a clinical picture simulating systemic lupus erythematosus including glomerulonephritis. In such patients hydrALAZINE should be discontinued unless the ...
-
PRECAUTIONSGeneral - Myocardial stimulation produced by hydrALAZINE can cause anginal attacks and ECG changes of myocardial ischemia. The drug has been implicated in the production of myocardial infarction ...
-
Adverse ReactionsAdverse reactions with hydrALAZINE are usually reversible when dosage is reduced. However, in some cases it may be necessary to discontinue the drug. The following adverse reactions have been ...
-
OVERDOSAGEAcute Toxicity: No deaths due to acute poisoning have been reported. Highest known dose survived: adults, 10 g orally. Oral LD50 in rats: 173 and 187 mg/kg. Signs and Symptoms: Signs and ...
-
DOSAGE AND ADMINISTRATIONInitiate therapy in gradually increasing dosages; adjust according to individual response. Start with 10 mg four times daily for the first 2 to 4 days, increase to 25 mg four times daily for the ...
-
HOW SUPPLIEDHydrALAZINE Hydrochloride Tablets, USP - 10 mg - round, convex, pink film-coated tablet engraved with HP above 1 on one side and plain on the other side - NDC 23155-001-01 Bottles of 100 w/ CRC - NDC ...
-
STORAGE AND HANDLINGStore at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELHydralazine Hydrochloride Tablets, USP, 10 mg, 100 count - Hydralazine Hydrochloride Tablets, USP, 25 mg, 100 count - Hydralazine Hydrochloride Tablets, USP, 50 mg, 100 ...
-
INGREDIENTS AND APPEARANCEProduct Information