Label: NYSTATIN AND TRIAMCINOLONE ACETONIDE- nystatin and triamcinolone acetonide ointment
- NDC Code(s): 21922-031-04, 21922-031-05, 21922-031-07
- Packager: Encube Ethicals Private Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
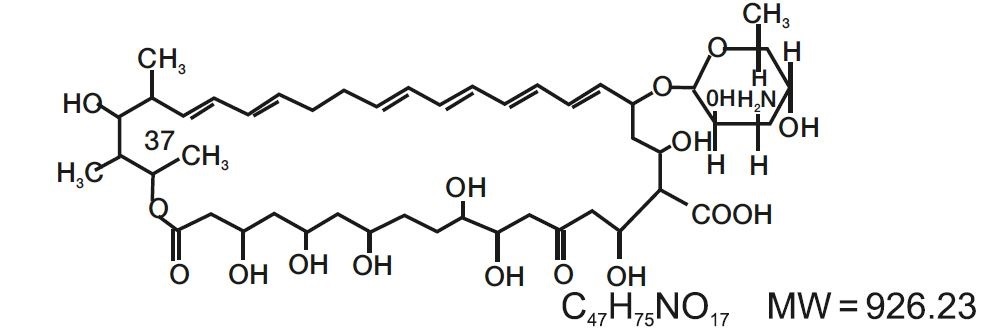
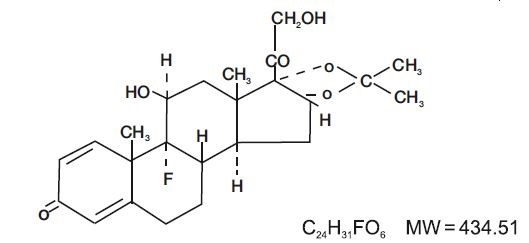
DESCRIPTIONNystatin and Triamcinolone Acetonide Ointment, USP for dermatologic use contain the antifungal agent nystatin and the synthetic corticosteroid triamcinolone acetonide. Nystatin ...
-
CLINICAL PHARMACOLOGYNystatin - Nystatin exerts its antifungal activity against a variety of pathogenic and nonpathogenic yeasts and fungi by binding to sterols in the cell membrane. The binding process renders the ...
-
INDICATIONS AND USAGENystatin and Triamcinolone Acetonide Ointment, USP is indicated for the treatment of cutaneous candidiasis; it has been demonstrated that the nystatin-steroid combination provides greater benefit ...
-
CONTRAINDICATIONSThese preparations are contraindicated in those patients with a history of hypersensitivity to any of their components.
-
PRECAUTIONSGeneral - Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and ...
-
ADVERSE REACTIONSA single case (approximately one percent of patients studied) of acneiform eruption occurred with use of combined nystatin and triamcinolone acetonide in clinical studies. Nystatin ...
-
OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see - PRECAUTIONS, General); however, acute overdosage and serious adverse effects ...
-
DOSAGE AND ADMINISTRATIONA thin film of Nystatin and Triamcinolone Acetonide Ointment, USP is usually applied to the affected areas twice daily in the morning and evening. The preparation should be discontinued if ...
-
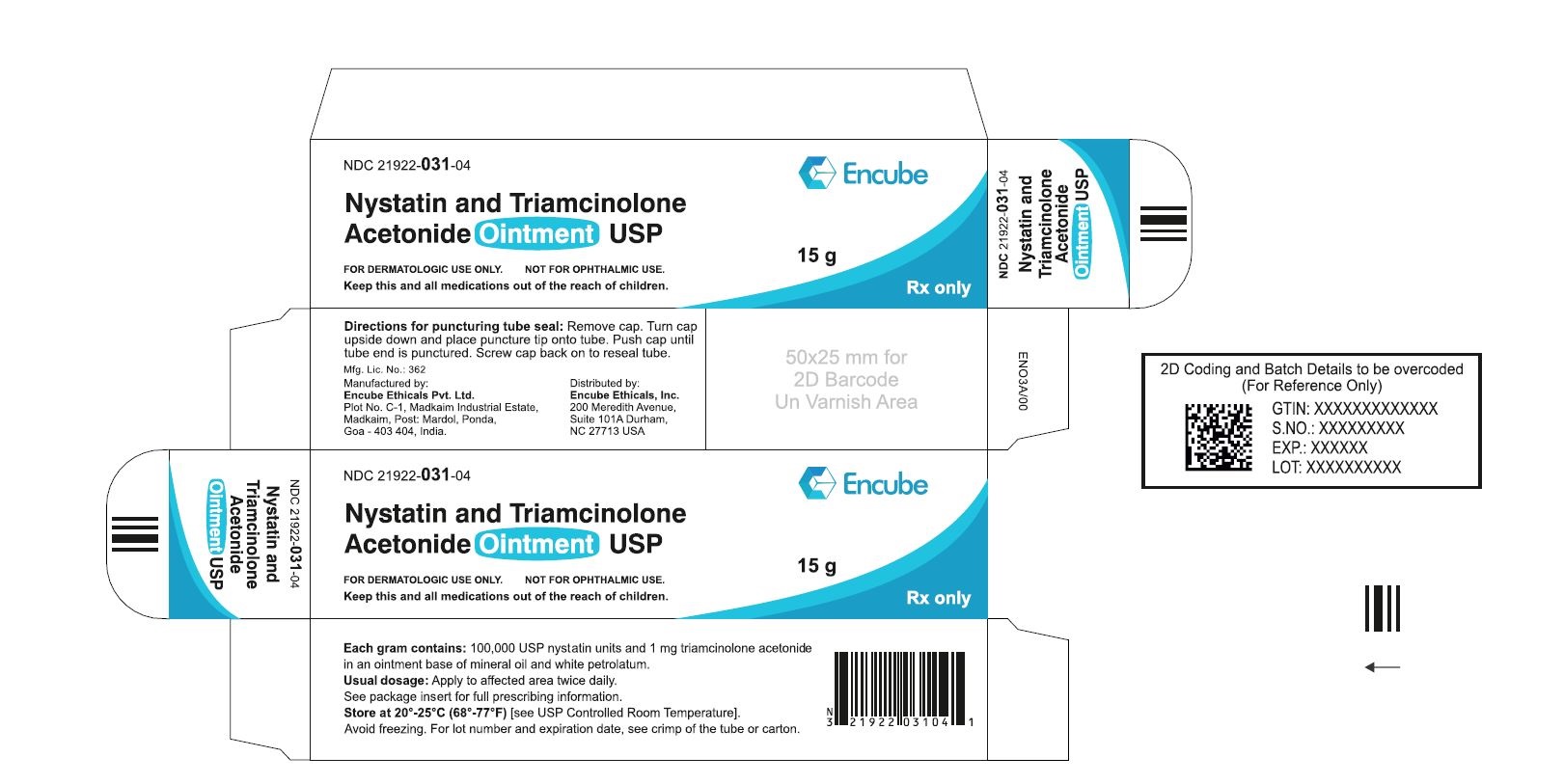
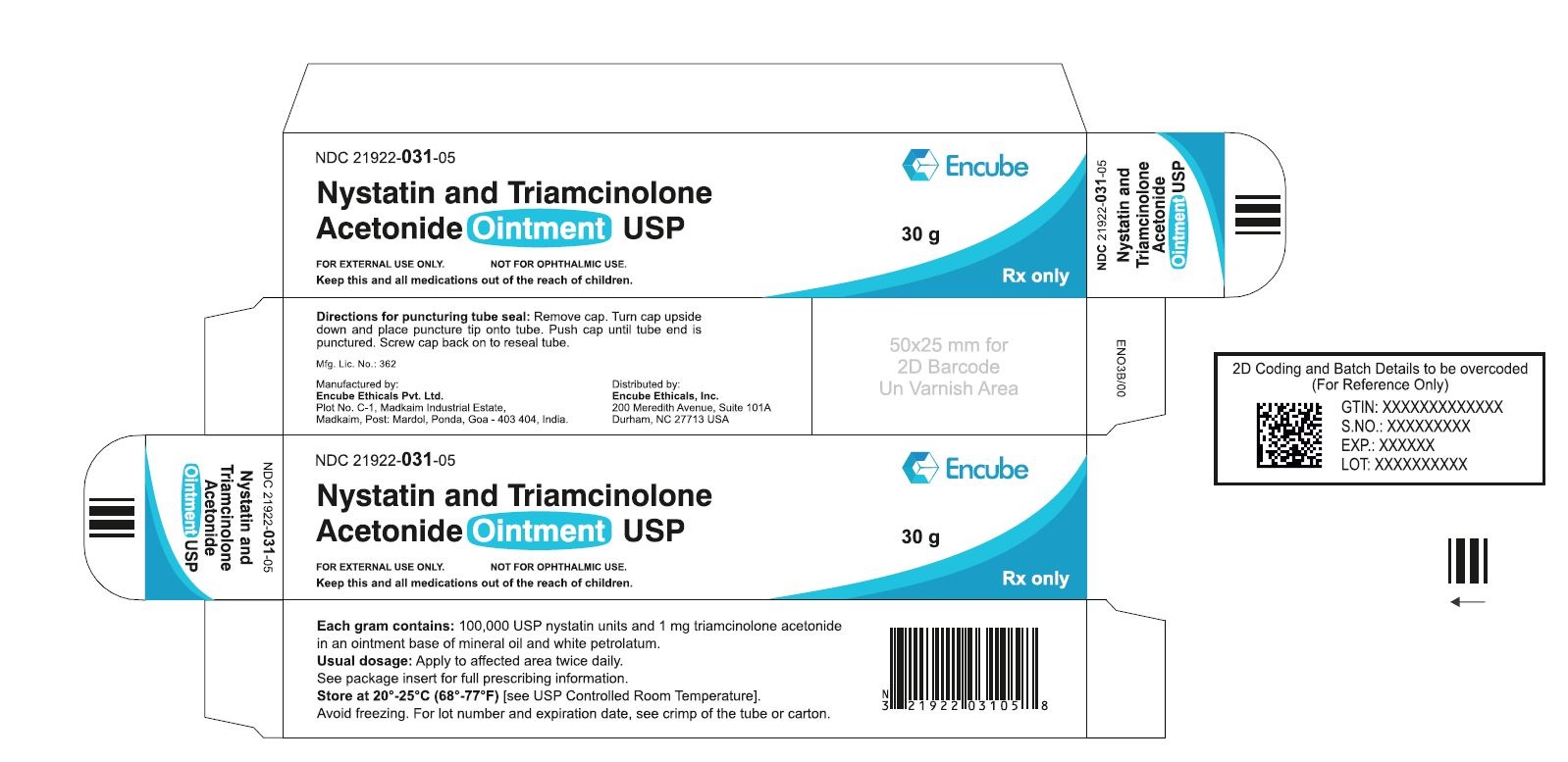
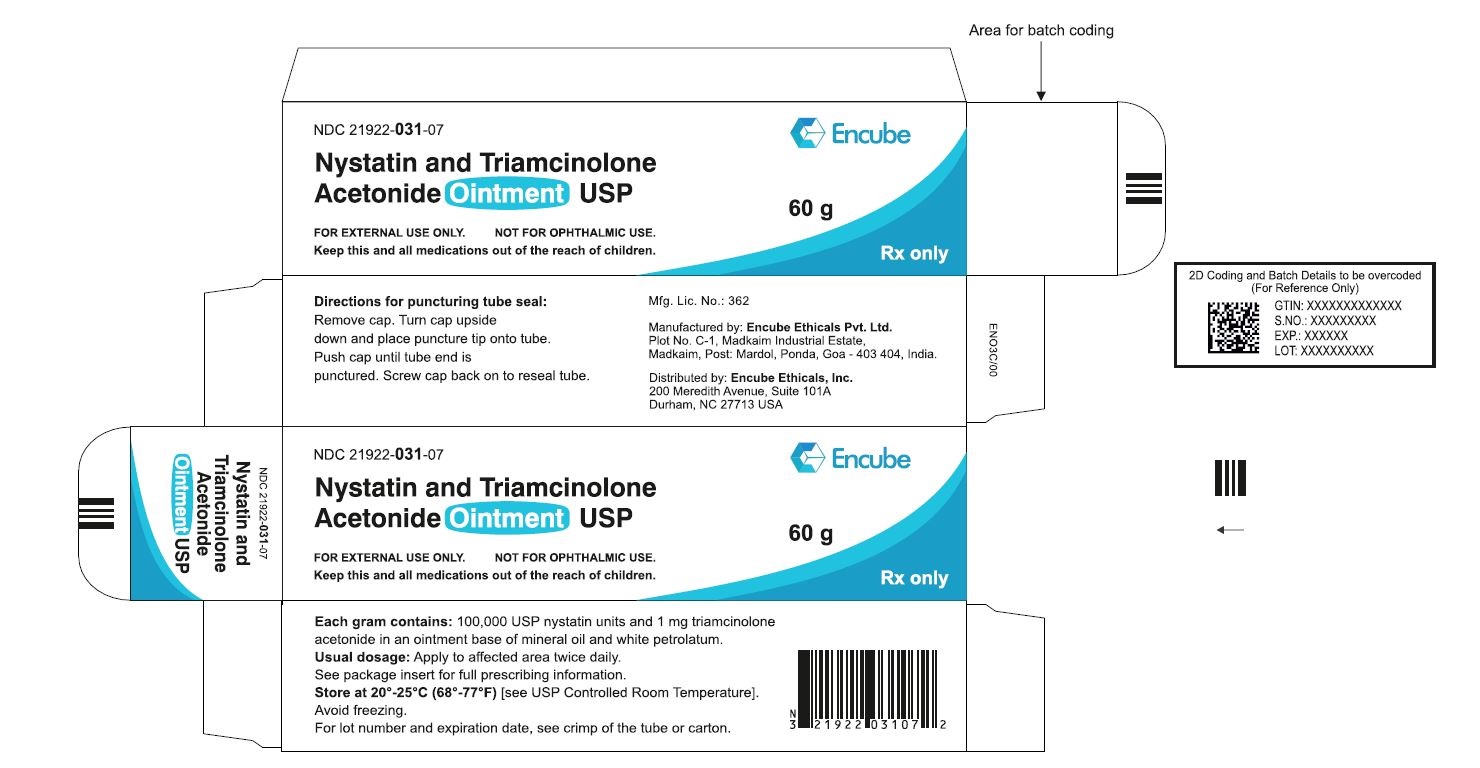
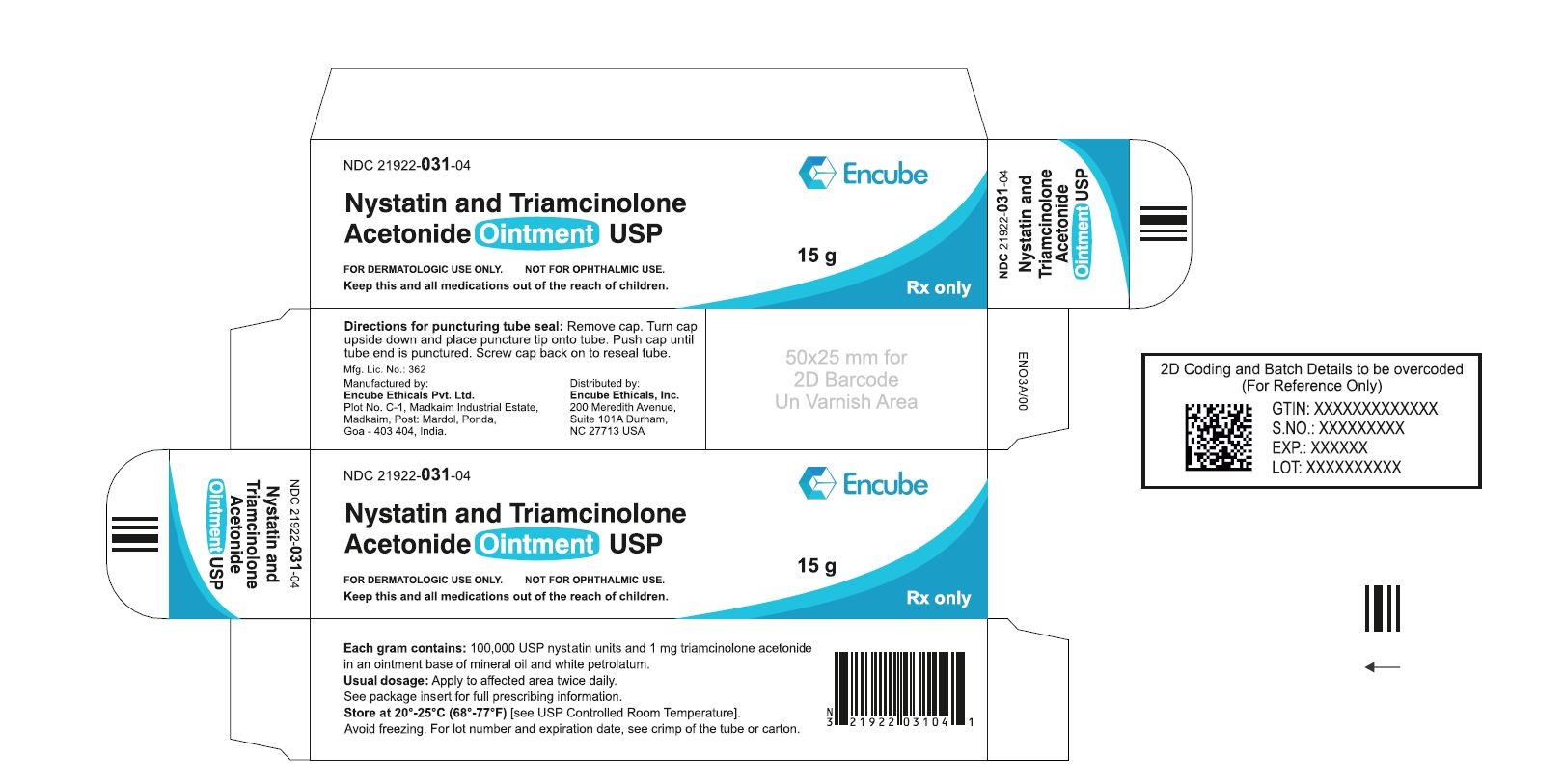
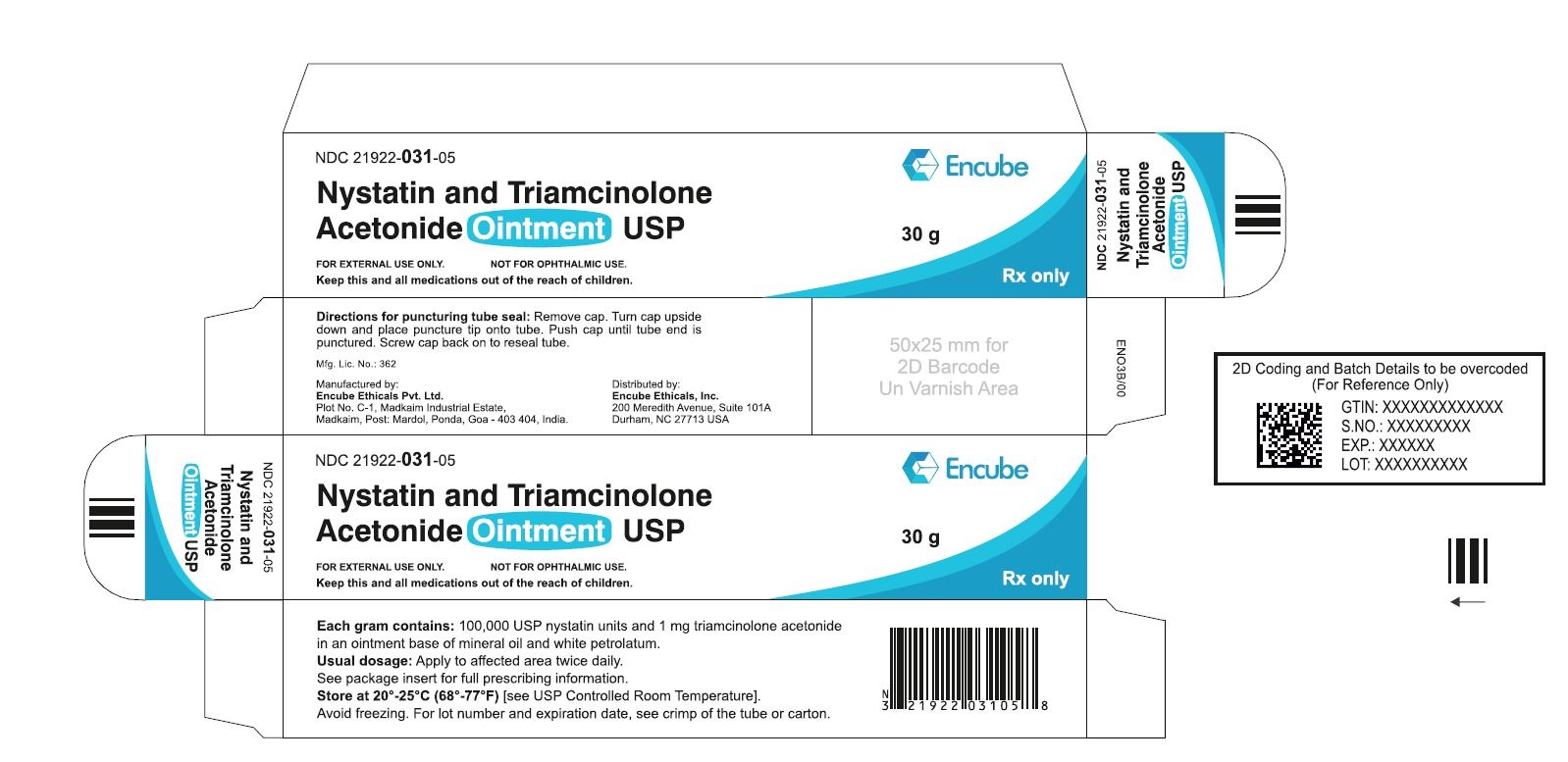
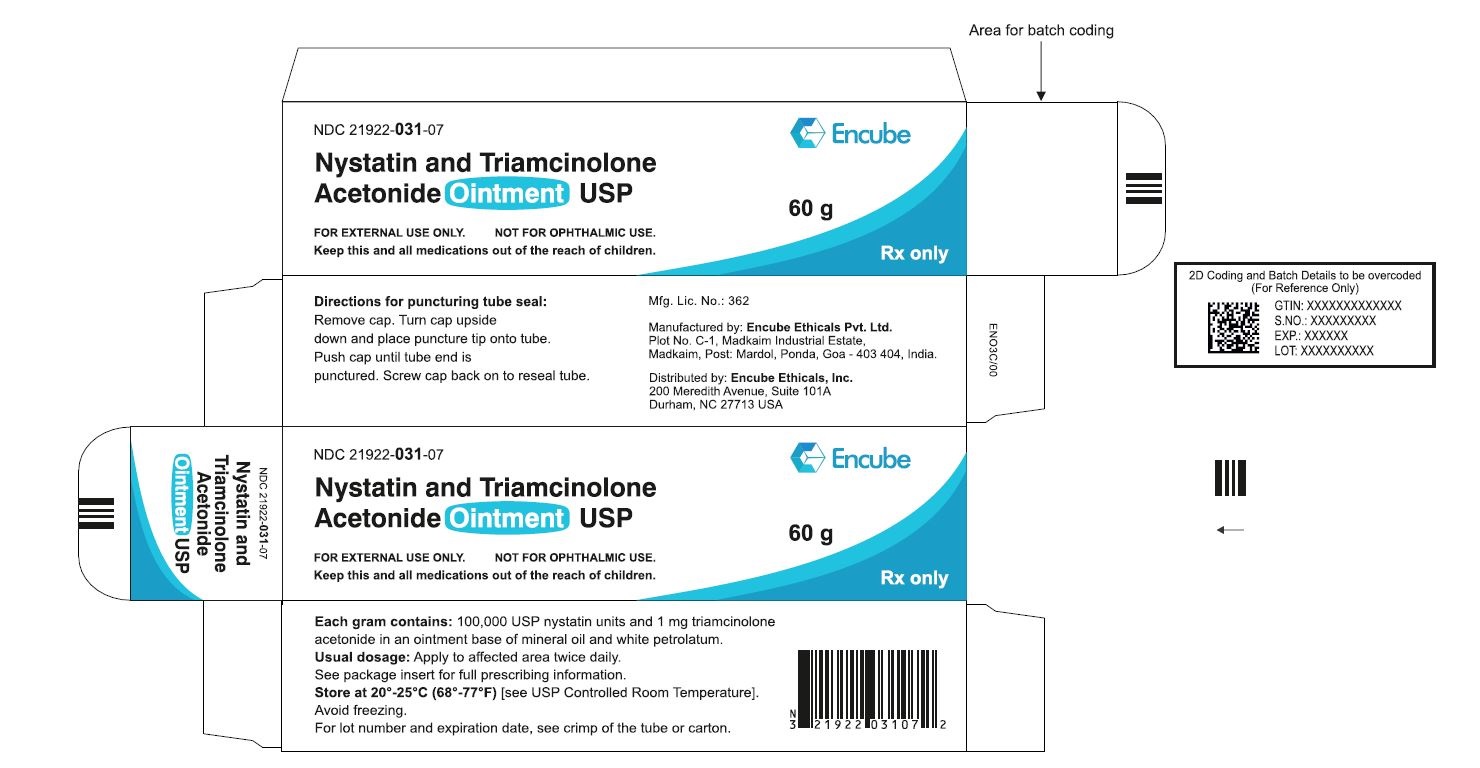
HOW SUPPLIEDNystatin and Triamcinolone Acetonide Ointment, USP is supplied in - 15g (NDC 21922-031-04), 30g (NDC 21922-031-05) and - 60g (NDC 21922-031-07) tubes ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 21922-031-04 Carton-15g - Nystatin and Triamcinolone Acetonide Ointment USP - FOR DERMATOLOGIC USE ONLY. NOT FOR OPHTHALMIC USE. Keep this and all medications out ...
-
INGREDIENTS AND APPEARANCEProduct Information