Label: MUPIROCIN cream
- NDC Code(s): 21922-029-04, 21922-029-05
- Packager: Encube Ethicals Private Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MUPIROCIN CREAM safely and effectively. See full prescribing information for MUPIROCIN CREAM. MUPIROCIN cream, for topical use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMupirocin Cream USP, 2% is indicated for the treatment of secondarily infected traumatic skin lesions (up to 10 cm in length or 100 cm - 2 in area) due to susceptible isolates of ...
-
2 DOSAGE AND ADMINISTRATIONFor Topical Use Only. Apply a small amount of mupirocin cream, with a cotton swab or gauze pad, to the affected area 3 times daily for 10 days. Cover the treated area with gauze dressing if ...
-
3 DOSAGE FORMS AND STRENGTHSMupirocin Cream USP, 2% m is a white cream that contains 20 mg (2% w/w) of mupirocin per gram in an oil- and water-based emulsion, supplied in 15-gram and 30-gram tubes.
-
4 CONTRAINDICATIONSMupirocin cream is contraindicated in patients with known hypersensitivity to mupirocin or any of the excipients of mupirocin cream.
-
5 WARNINGS AND PRECAUTIONS5.1 Severe Allergic Reactions - Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash, have been reported in patients treated with formulations of ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Severe Allergic Reactions - [see Warnings and Precautions ( 5.1)] Eye Irritation ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient human data to establish whether there is a drug-associated risk with mupirocin cream in pregnant women. Systemic absorption of mupirocin ...
-
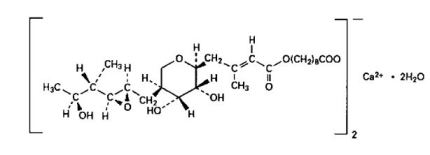
11 DESCRIPTIONMupirocin Cream USP, 2% contains the dihydrate crystalline calcium hemi-salt of the RNA synthetase inhibitor antibacterial, mupirocin. Chemically, it is (α E,2 - S,3 - R,4 - R,5 - S)-5-[(2 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Mupirocin is an RNA synthetase inhibitor antibacterial - [see Microbiology ( 12.4)]. 12.3 Pharmacokinetics ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential of mupirocin calcium have not been conducted. Results of the ...
-
14 CLINICAL STUDIESThe efficacy of topical mupirocin cream for the treatment of secondarily infected traumatic skin lesions (e.g., lacerations, sutured wounds, and abrasions not more than 10 cm in length or 100 cm ...
-
15 REFERENCES1. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement. CLSI document M100-S26. Clinical ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMupirocin Cream USP, 2% is a white cream that contains 20 mg (2% w/w) of mupirocin per gram in an oil- and water-based emulsion. Mupirocin Cream USP, 2% is supplied in 15-gram and 30-gram ...
-
17 PATIENT COUNSELING INFORMATIONdvise the patient to read the FDA-approved patient labeling (Patient Information). Advise the patient to administer mupirocin cream as follows: Use mupirocin cream only as directed by the ...
-
Patient InformationPATIENT INFORMATION - MUPIROCIN (mue pir'oh sin) CREAM, for topical use - What is mupirocin cream? Mupirocin cream is a prescription medicine used on the skin (topical use) to treat ...
-
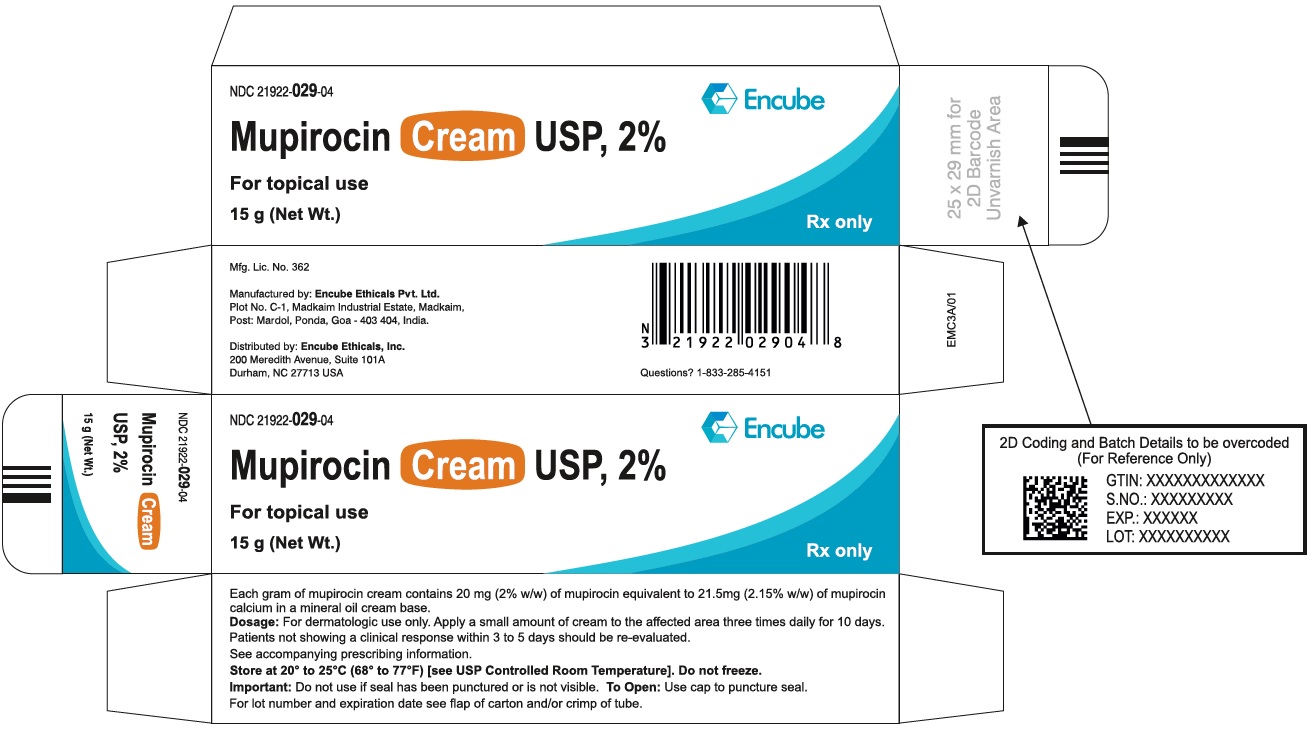
PACKAGE/LABEL PRINCIPAL DISPLAY PANELTube Label - NDC 21922-029-04 (Mupirocin Cream USP, 2% - Tube-15 g) NDC 21922-029-04 - Mupirocin Cream USP, 2% For topical use - 15 g (Net Wt.) Rx only - Carton Label - NDC 21922-029-04 ...
-
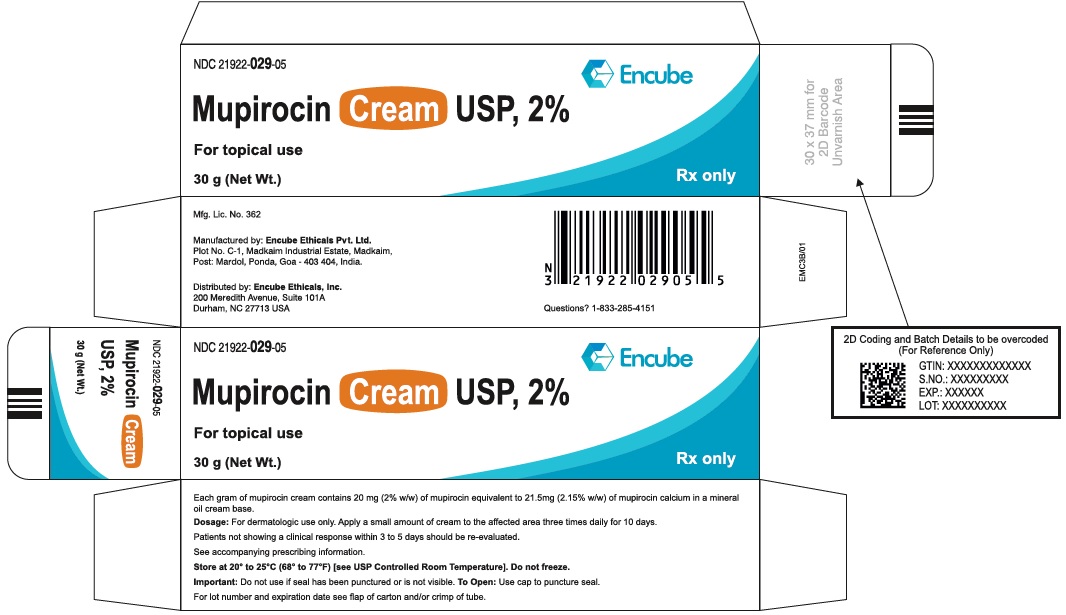
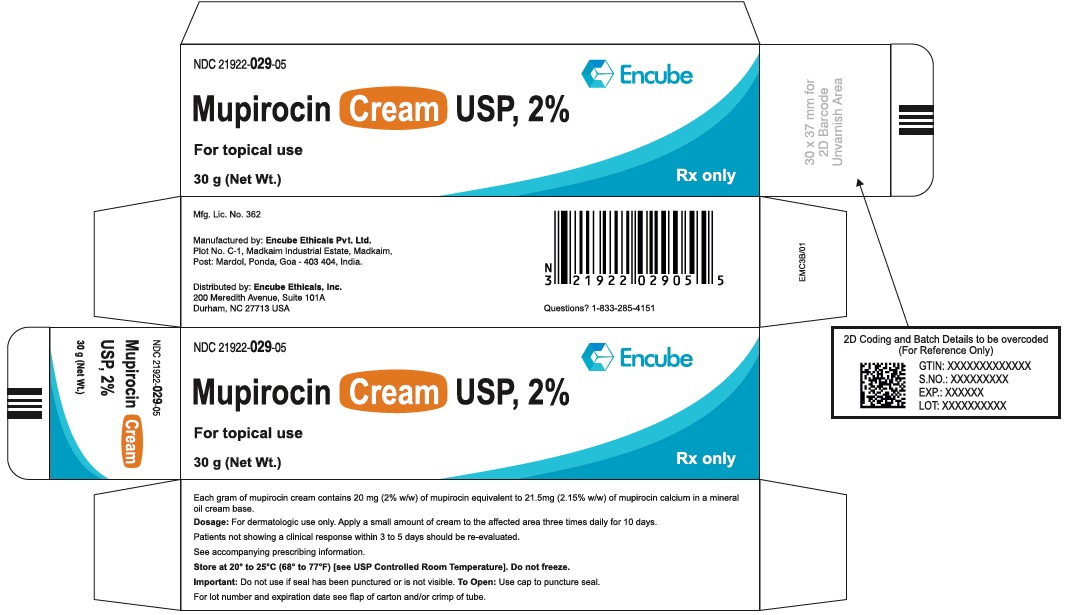
PACKAGE/LABEL PRINCIPAL DISPLAY PANELube Label - NDC 21922-029-05 (Mupirocin Cream USP, 2% - Tube-30 g) NDC 21922-029-05 - Mupirocin Cream USP, 2% For topical use - 30 g (Net Wt.) Rx only - Carton Label - NDC 21922-029-05 ...
-
INGREDIENTS AND APPEARANCEProduct Information