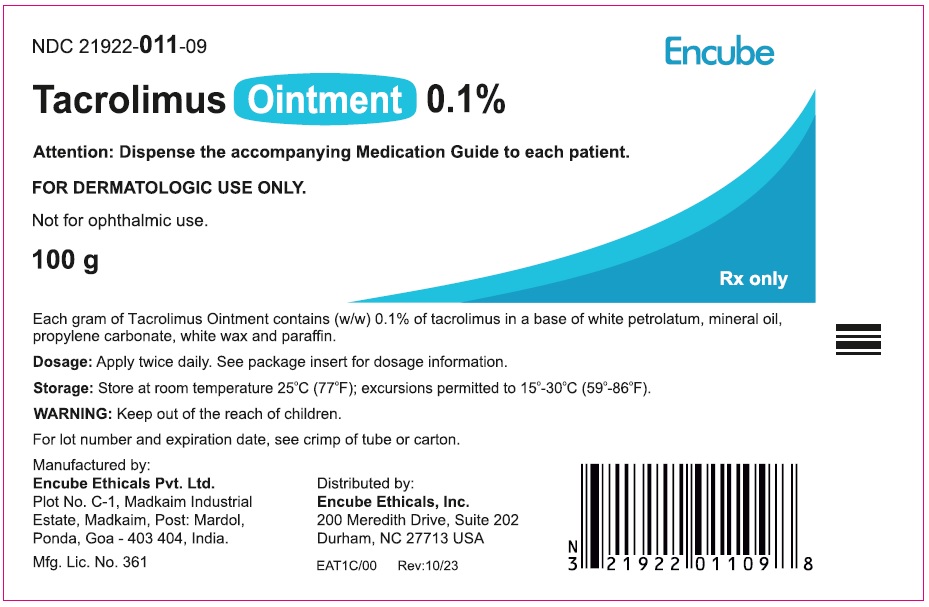
Label: TACROLIMUS- tacrolimus ointment 0.1% ointment
- NDC Code(s): 21922-011-05, 21922-011-07, 21922-011-09
- Packager: Encube Ethicals Private Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
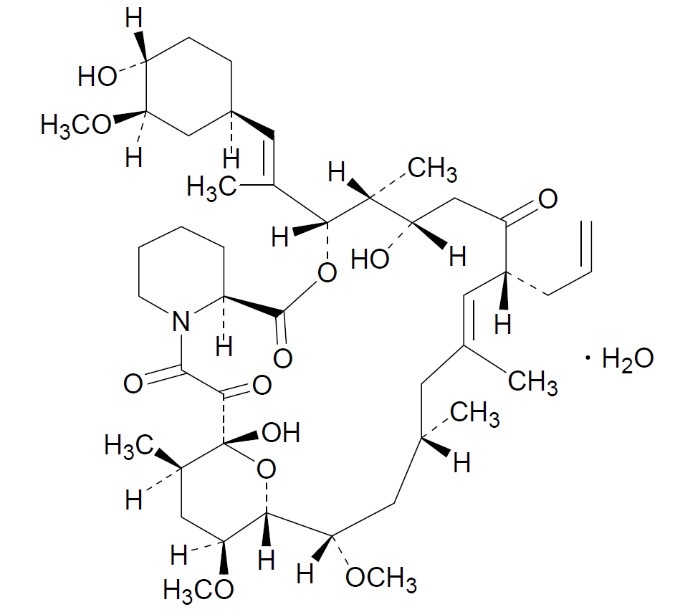
DESCRIPTIONTacrolimus ointment contains tacrolimus, a macrolide immunosuppressant produced by Streptomyces tsukubaensis. It is for topical dermatologic use only. Chemically, tacrolimus is designated as ...
-
CLINICAL PHARMACOLOGYMechanism of Action - The mechanism of action of tacrolimus in atopic dermatitis is not known. While the following have been observed, the clinical significance of these observations in atopic ...
-
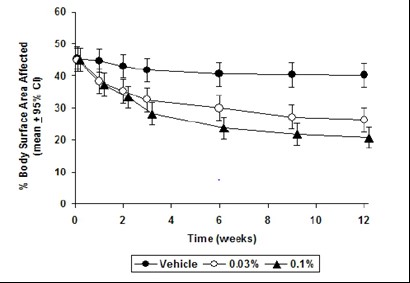
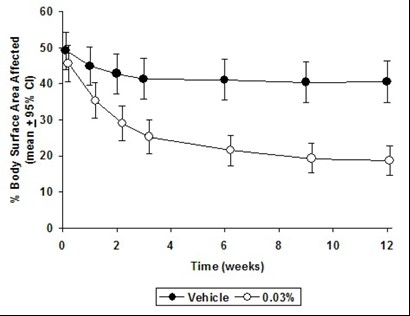
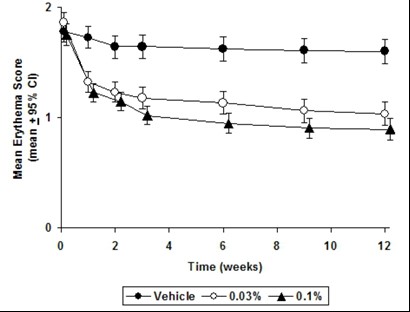
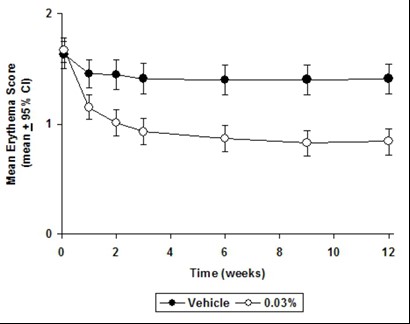
CLINICAL STUDIESThree randomized, double-blind, vehicle-controlled, multi-center, phase 3 studies were conducted to evaluate tacrolimus ointment for the treatment of patients with moderate to severe atopic ...
-
INDICATIONS AND USAGETacrolimus ointment, both 0.03% and 0.1% for adults, and only 0.03% for children aged 2 to 15 years, is indicated as second-line therapy for the short-term and non-continuous chronic treatment of ...
-
CONTRAINDICATIONSTacrolimus ointment is contraindicated in patients with a history of hypersensitivity to tacrolimus or any other component of the ointment.
-
BOXED WARNING
(What is this?)
WARNING
Long-term Safety of Topical Calcineurin Inhibitors Has Not Been Established
Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including tacrolimus ointment.
Therefore:
Close
• Continuous long-term use of topical calcineurin inhibitors, including tacrolimus ointment, in any age group should be avoided, and application limited to areas of involvement with atopic dermatitis.
• Tacrolimus ointment is not indicated for use in children less than 2 years of age. Only 0.03% tacrolimus ointment is indicated for use in children 2-15 years of age. -
WARNINGSProlonged systemic use of calcineurin inhibitors for sustained immunosuppression in animal studies and transplant patients following systemic administration has been associated with an increased ...
-
PRECAUTIONSGeneral - The use of tacrolimus ointment should be avoided on pre-malignant and malignant skin conditions. Some malignant skin conditions, such as cutaneous T-cell lymphoma (CTCL), may mimic ...
-
ADVERSE REACTIONSNo phototoxicity and no photoallergenicity were detected in clinical studies with 12 and 216 normal volunteers, respectively. One out of 198 normal volunteers showed evidence of sensitization in a ...
-
OVERDOSAGETacrolimus ointment is not for oral use. Oral ingestion of tacrolimus ointment may lead to adverse effects associated with systemic administration of tacrolimus. If oral ingestion occurs, medical ...
-
DOSAGE AND ADMINISTRATIONADULT - Tacrolimus ointment 0.1% Apply a thin layer of tacrolimus ointment to the affected skin twice daily. The minimum amount should be rubbed in gently and completely to control signs and ...
-
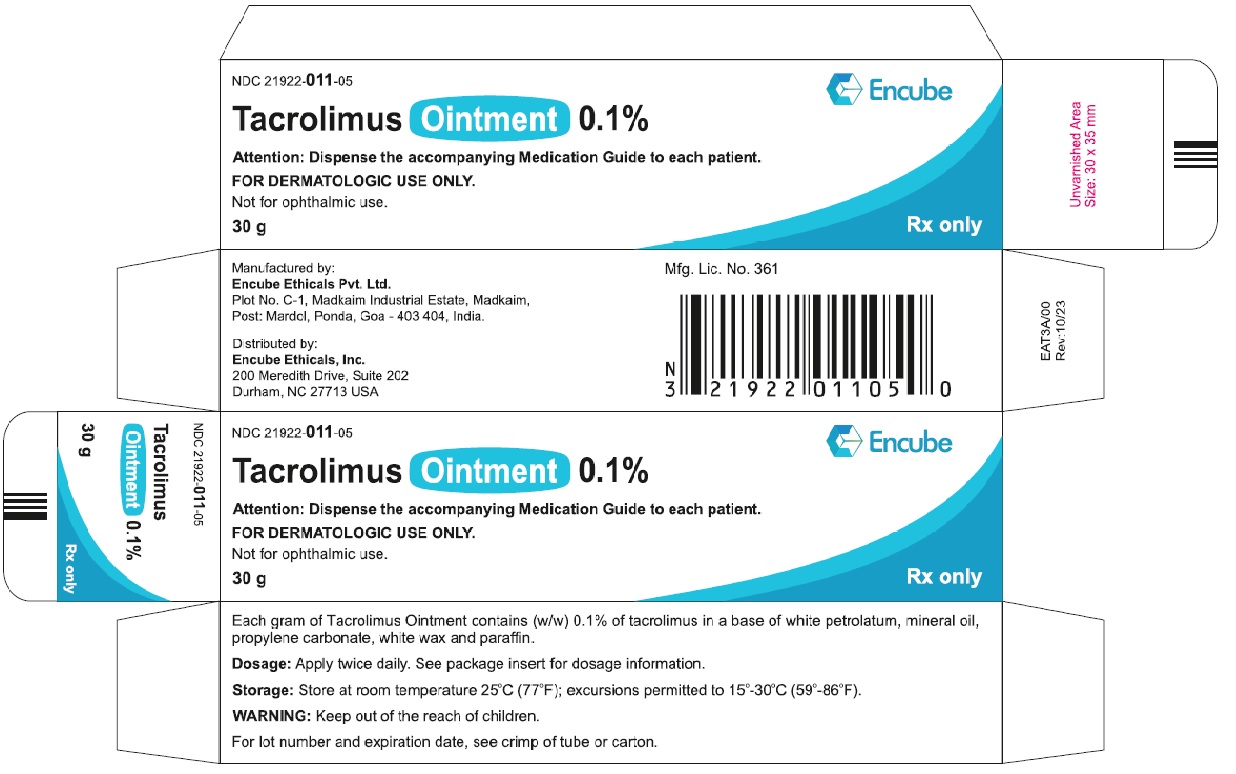
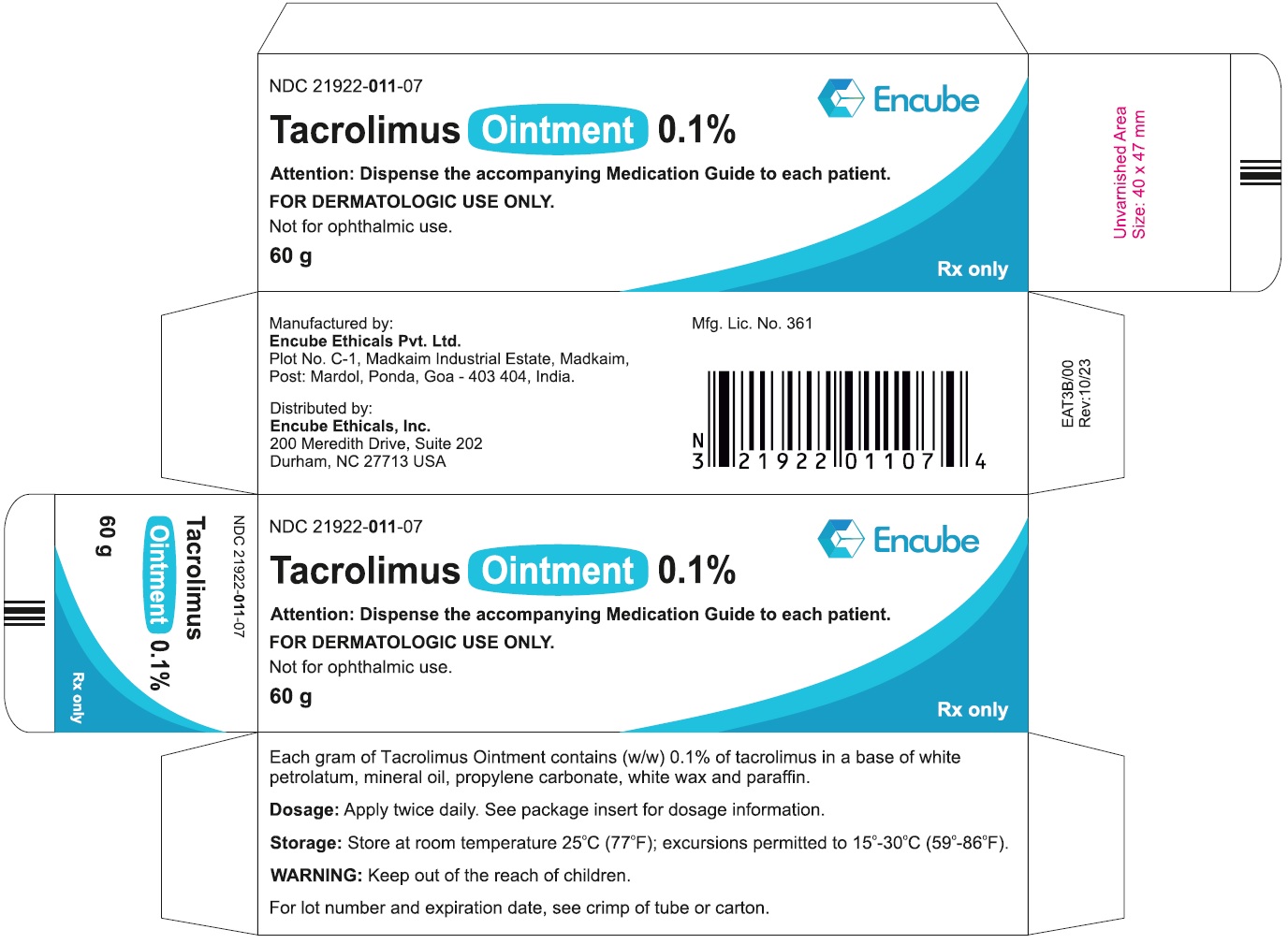
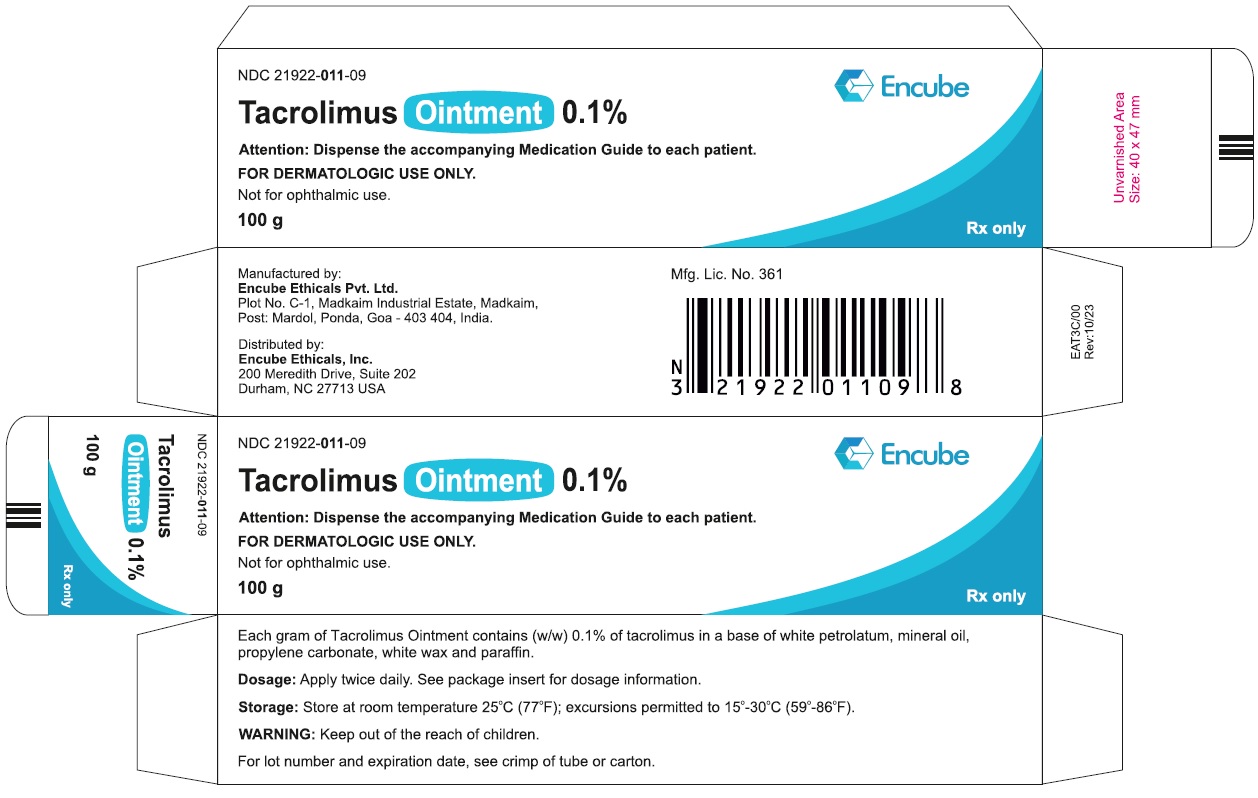
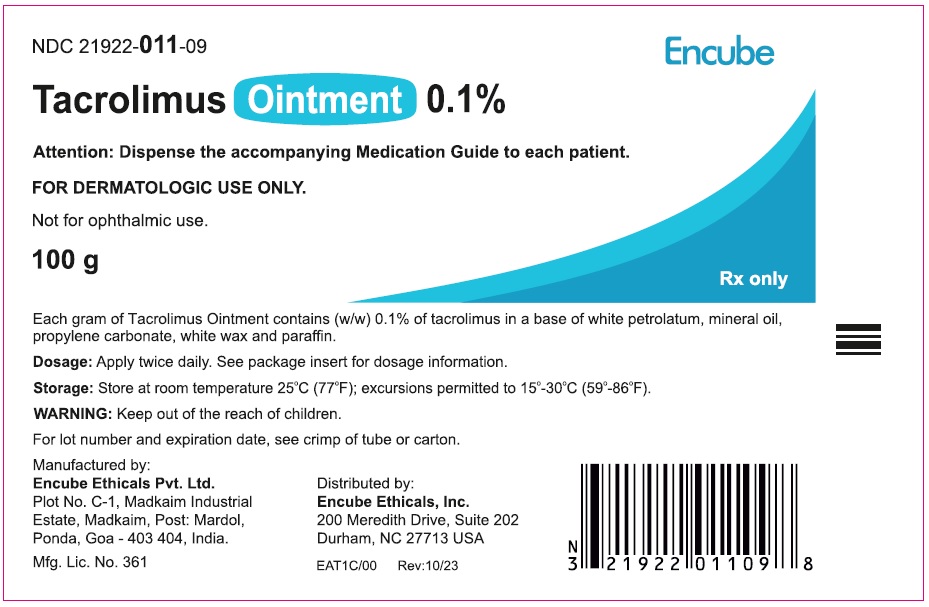
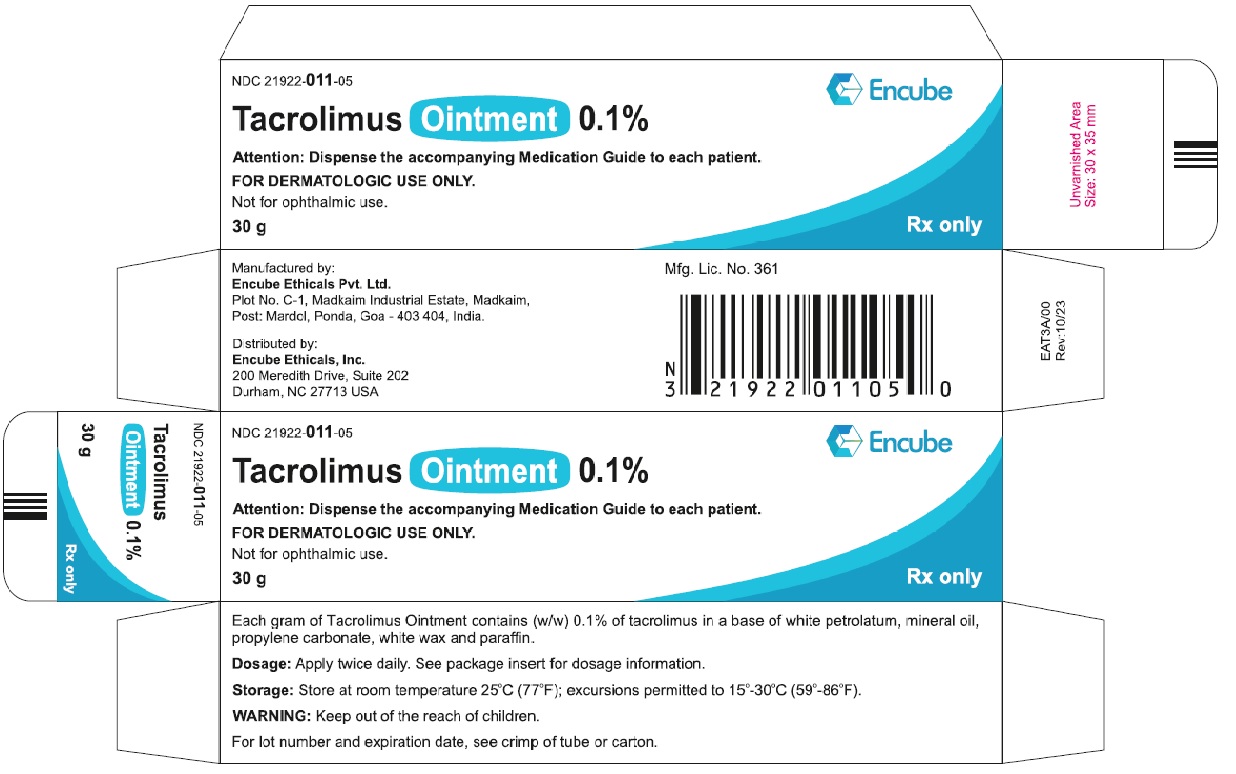
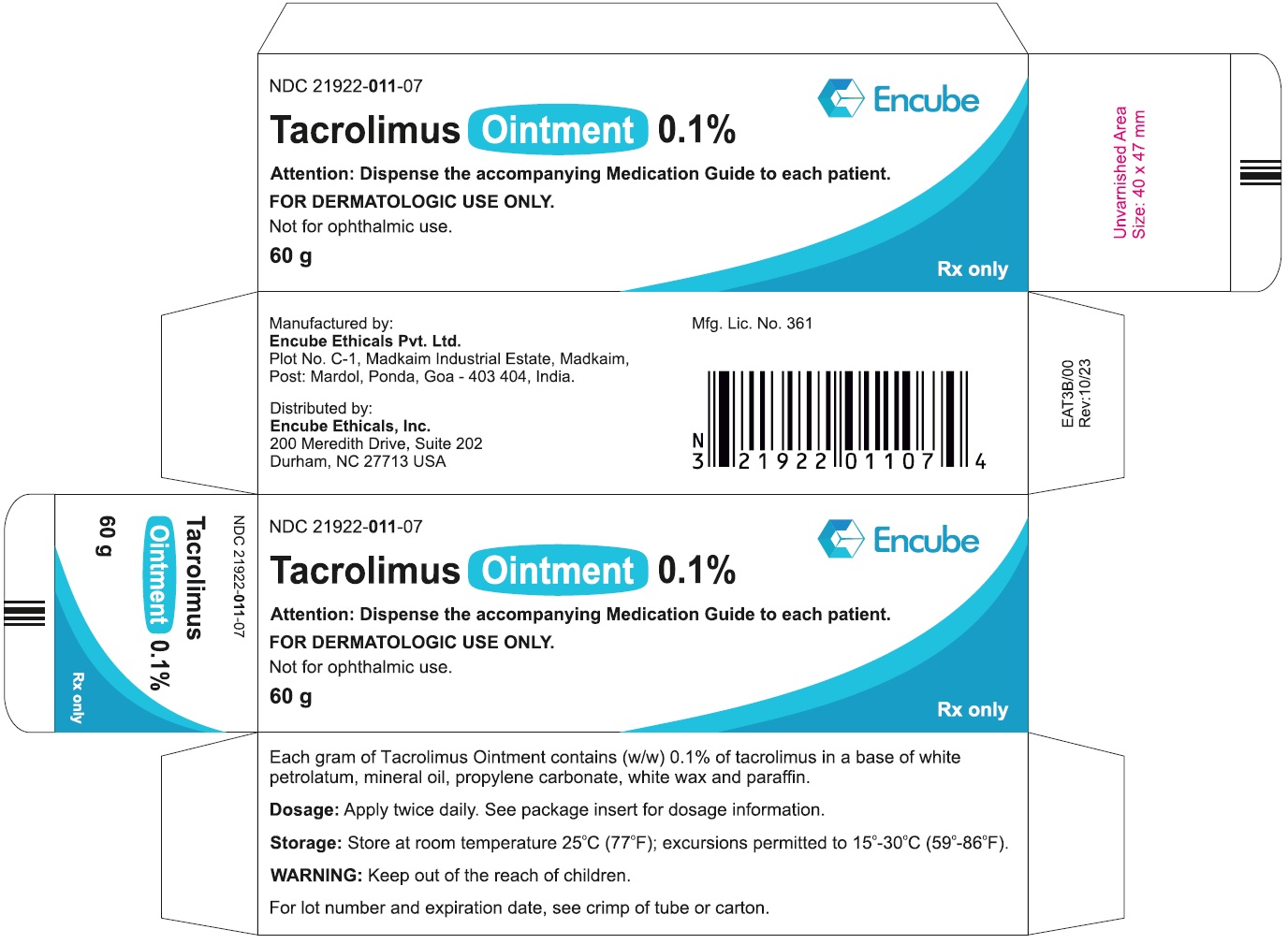
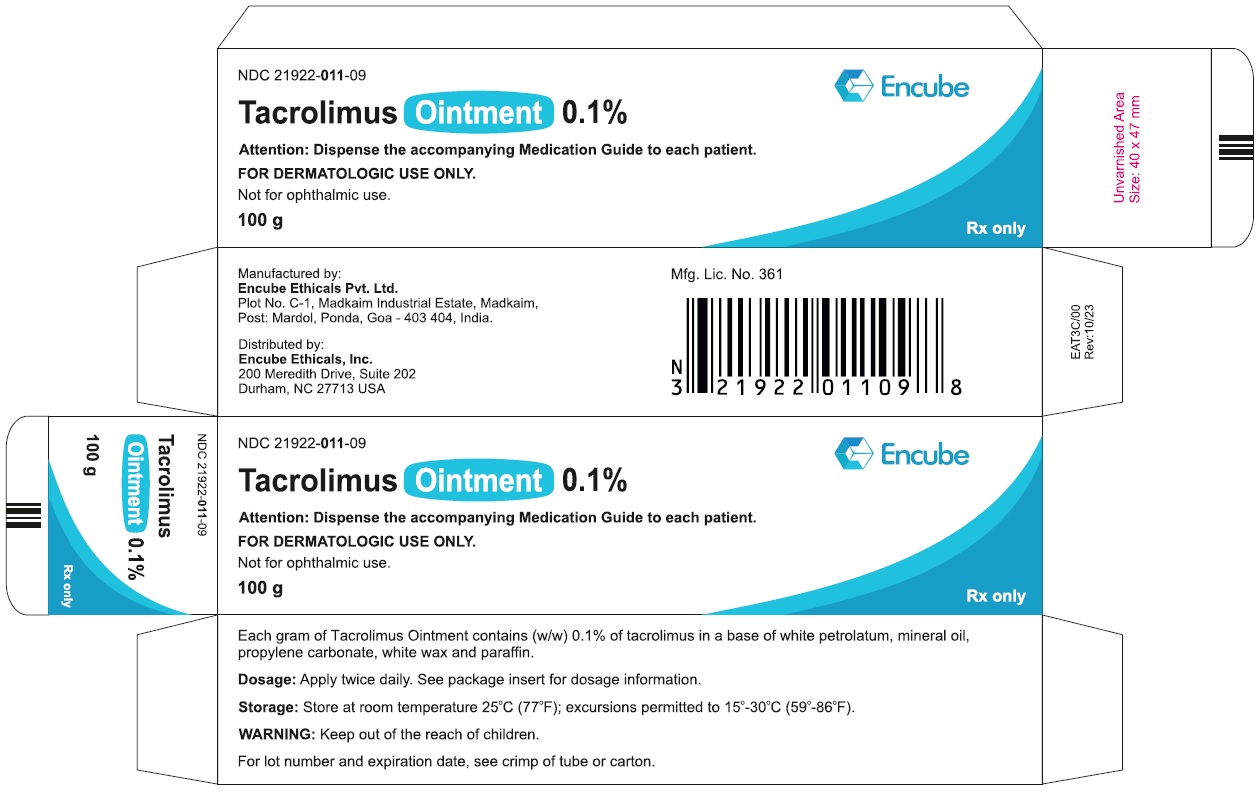
HOW SUPPLIEDTacrolimus ointment 0.1% is supplied in the following tube sizes: Tacrolimus Ointment 0.1% NDC21922-011-05 - 30 gram laminate tube - NDC21922-011-07 - 60 gram laminate tube - NDC21922-011-09 - 100 ...
-
MEDICATION GUIDETacrolimus (ta kroe' li mus) Ointment 0.1% Read the Medication Guide every time you or a family member gets tacrolimus ointment. There may be new information. This Medication Guide does ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELDraft Carton Label - 30g - NDC 21922-011-05 - Tacrolimus Ointment 0.1% Attention: Dispense the accompanying Medication Guide to each patient. FOR DERMATOLOGIC USE ONLY. Not for ophthalmic ...
-
INGREDIENTS AND APPEARANCEProduct Information