Label: CLINDAMYCIN PHOAPHATE TOPICAL SOLUTION USP, 1%- clindamycin phosphate topical solution usp, 1% solution
- NDC Code(s): 21922-002-01, 21922-002-21
- Packager: Encube Ethicals Private Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
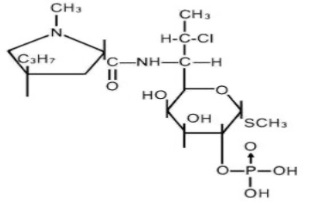
DESCRIPTIONClindamycin phosphate topical solution USP, 1% contain clindamycin phosphate, USP, at a concentration equivalent to 10 mg clindamycin per milliliter. Clindamycin phosphate is a water soluble ...
-
CLINICAL PHARMACOLOGYMechanism of Action: The Mechanism of action of clindamycin in treating acne vulgaris is unknown. Pharmacokinetics - Following multiple topical applications of clindamycin phosphate at a ...
-
INDICATIONS AND USAGEClindamycin phosphate topical solution USP, 1% is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician ...
-
CONTRAINDICATIONSClindamycin phosphate topical solution USP, 1% is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional ...
-
WARNINGSOrally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption ...
-
PRECAUTIONSGeneral - Clindamycin phosphate topical solution USP, 1% contains an alcohol base which will cause burning and irritation of the eye. In the event of accidental contact with sensitive surfaces ...
-
ADVERSE REACTIONSIn 18 clinical studies of various formulations of clindamycin phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent ...
-
OVERDOSAGETopically applied clindamycin phosphate topical solution USP, 1% can be absorbed in sufficient amounts to produce systemic effects (see WARNINGS).
-
DOSAGE AND ADMINISTRATIONApply a thin film of Clindamycin phosphate topical solution USP, 1% twice daily to affected area. Keep container tightly closed.
-
HOW SUPPLIEDClindamycin phosphate topical solution USP, 1% containing clindamycin phosphate equivalent to 10 mg clindamycin per millilitre is available in the following size: 60 mL Bottle – NDC ...
-
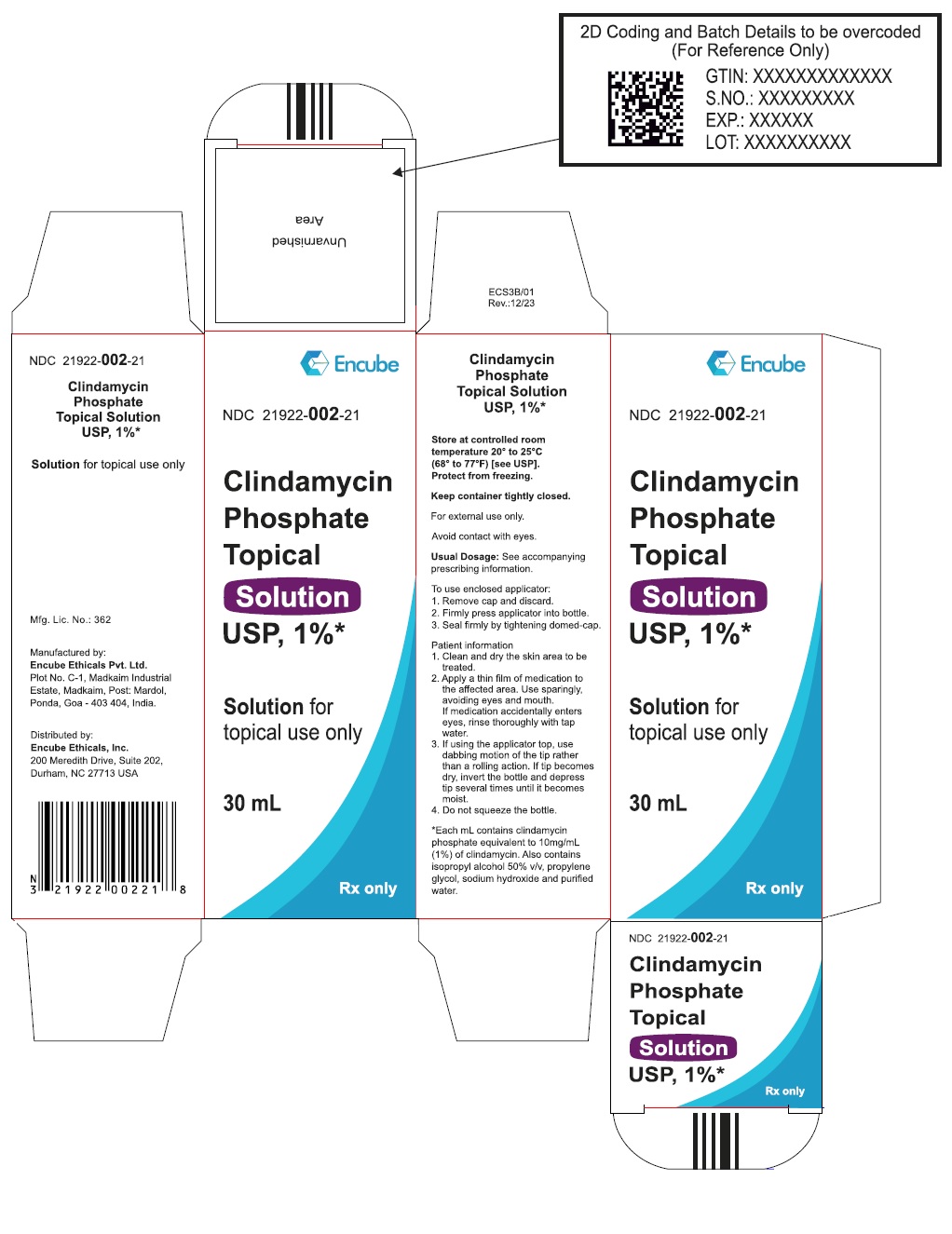
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 60ML BOTTLE CARTON - NDC - 21922-002-01 - Clindamycin Phosphate Topical Solution USP, 1%* Solution for topical use only - 60 mL - Rx only - Store at ...
-
INGREDIENTS AND APPEARANCEProduct Information