Label: VANCOMYCIN HYDROCHLORIDE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 17478-741-02, 17478-742-02 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VANCOMYCIN HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for VANCOMYCIN HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Vancomycin hydrochloride capsule is indicated for the treatment of Clostridioides difficile-associated diarrhea. Vancomycin hydrochloride capsule is also used for the treatment of enterocolitis ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Adults - Vancomycin hydrochloride capsule are used in treating C. difficile-associated diarrhea and staphylococcal enterocolitis. C. difficile-associated diarrhea: The recommended dose is ...
-
3 DOSAGE FORMS AND STRENGTHS
Vancomycin Hydrochloride Capsule 125 mg (equivalent to vancomycin) capsules have an opaque blue cap and opaque brown body imprinted with “741” on the cap and “125 mg” on the body in white ...
-
4 CONTRAINDICATIONS
Vancomycin hydrochloride capsule is contraindicated in patients with known hypersensitivity to vancomycin.
-
5 WARNINGS AND PRECAUTIONS
5.1 Oral Use Only - Vancomycin hydrochloride capsule for the treatment of colitis is for oral use only and is not systemically absorbed. Vancomycin hydrochloride capsule must be given orally for ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
No drug interaction studies have been conducted.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Systemic absorption of vancomycin is low following oral administration of vancomycin hydrochloride capsule; however, absorption may vary depending on various ...
-
10 OVERDOSAGE
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to ...
-
11 DESCRIPTION
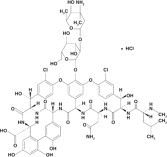
Vancomycin Hydrochloride Capsule, USP for oral administration contain chromatographically purified vancomycin hydrochloride, a tricyclic glycopeptide antibiotic derived from Amycolatopsis ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Vancomycin is an antibacterial drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Vancomycin is poorly absorbed after oral administration. During multiple ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term carcinogenesis studies in animals have been conducted. At concentrations up to 1000 mcg/mL, vancomycin had no mutagenic ...
-
14 CLINICAL STUDIES
14.1 Diarrhea Associated with Clostridioides difficile - In two trials, vancomycin hydrochloride capsule 125 mg orally four times daily for 10 days was evaluated in 266 adult subjects with C ...
-
15 REFERENCES
Byrd RA., Gries CL, Buening M.: Developmental Toxicology Studies of Vancomycin Hydrochloride Administered Intravenously to Rats and Rabbits. Fundam Appl Toxicol 1994; 23: 590-597.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Vancomycin Hydrochloride Capsules, USP are available in: The 125 mg* capsules have an opaque blue cap and opaque brown body imprinted with "741" on the cap and "125 mg" on the body in white ink ...
-
17 PATIENT COUNSELING INFORMATION
Severe Dermatologic Reactions - Advise patients about the signs and symptoms of serious skin manifestations. Instruct patients to stop taking Vancomycin hydrochloride capsule immediately and ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Container Label: NDC 17478-741-02 - Vancomycin HCl - Capsule, USP - 125 mg* *Equiv. to 125 mg Vancomycin
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Carton Label: NDC 17478-741-02 Akorn - Vancomycin Hydrochloride - Capsules, USP - 125 mg* *Equivalent to 125 mg vancomycin - Not a Child Resistant ...
-
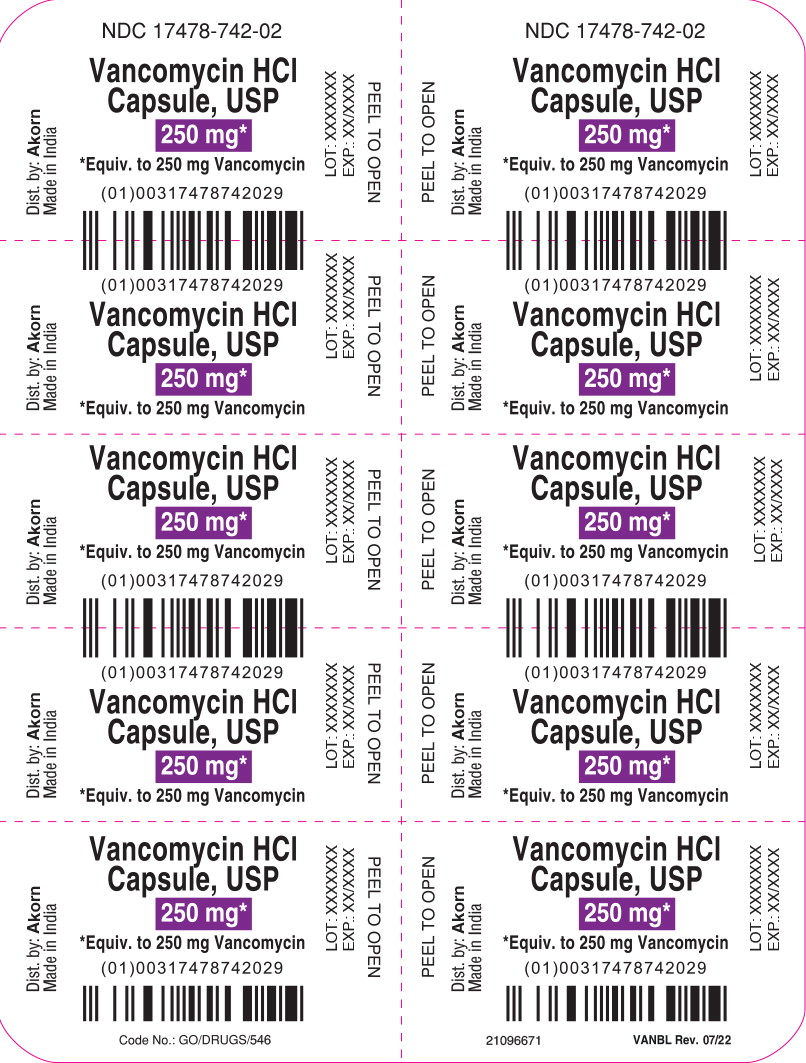
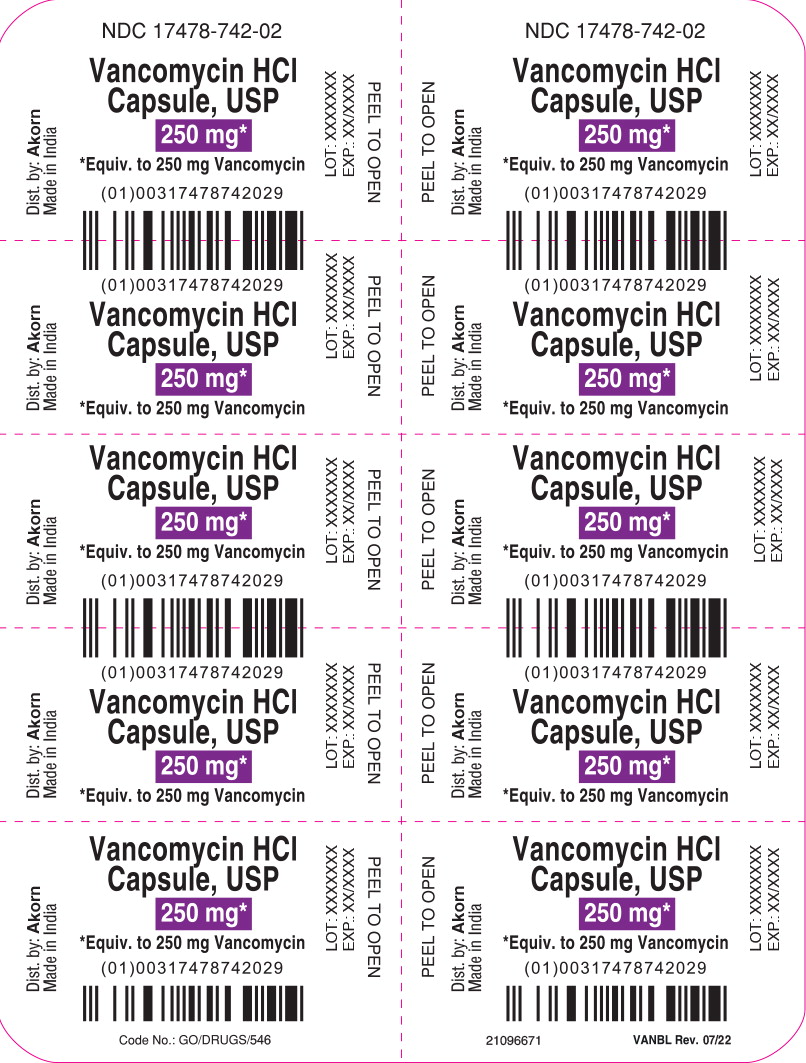
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Container Label: NDC 17478-742-02 - Vancomycin HCl - Capsule, USP - 250 mg* *Equiv. to 250 mg Vancomycin
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Carton Label: NDC 17478-742-02 Akorn - Vancomycin Hydrochloride - Capsules, USP - 250 mg* *Equivalent to 250 mg vancomycin - Not a Child Resistant ...
-
INGREDIENTS AND APPEARANCEProduct Information