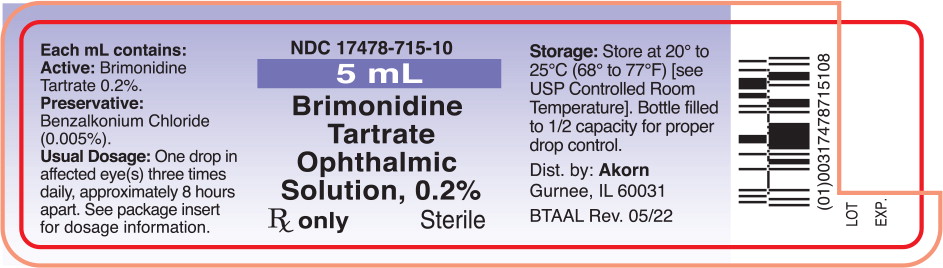
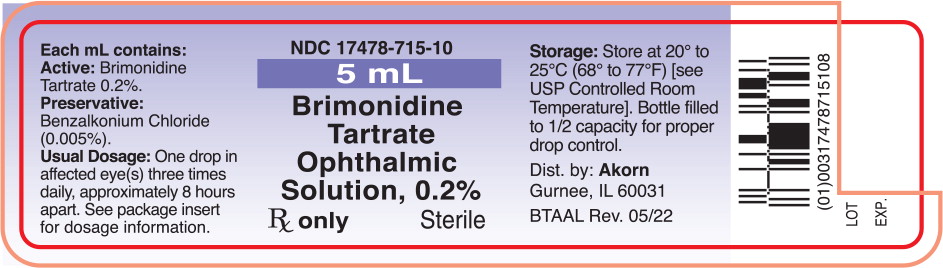
Label: BRIMONIDINE- brimonidine tartrate solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 17478-715-10, 17478-715-11, 17478-715-12 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BRIMONIDINE TARTRATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for BRIMONIDINE TARTRATE OPHTHALMIC ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Brimonidine tartrate ophthalmic solution 0.2% is indicated for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. The IOP lowering efficacy of ...
-
2 DOSAGE AND ADMINISTRATION
The recommended dose is one drop of brimonidine tartrate ophthalmic solution 0.2% in the affected eye(s) three times daily, approximately 8 hours apart. Brimonidine tartrate ophthalmic solution ...
-
3 DOSAGE FORMS AND STRENGTHS
Solution containing 2 mg/mL brimonidine tartrate.
-
4 CONTRAINDICATIONS
4.1 Neonates and Infants (under the age of 2 years) Brimonidine tartrate ophthalmic solution is contraindicated in neonates and infants (under the age of 2 years) [see Use in Specific ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Potentiation of Vascular Insufficiency - Brimonidine tartrate ophthalmic solution may potentiate syndromes associated with vascular insufficiency. Brimonidine tartrate ophthalmic solution ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Potentiation of Vascular Insufficiency [see Warnings and Precautions (5.1)] Severe Cardiovascular Disease [see ...
-
7 DRUG INTERACTIONS
7.1 Antihypertensives/Cardiac Glycosides - Because brimonidine tartrate ophthalmic solution may reduce blood pressure, caution in using drugs such as antihypertensives and/or cardiac glycosides ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Category B: Teratogenicity studies have been performed in animals. Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in ...
-
10 OVERDOSAGE
Very limited information exists on accidental ingestion of brimonidine in adults; the only adverse reaction reported to date has been hypotension. Symptoms of brimonidine overdose have been ...
-
11 DESCRIPTION
Brimonidine tartrate ophthalmic solution 0.2%, sterile, is a relatively selective alpha-2 adrenergic receptor agonist (topical intraocular pressure lowering agent). The structural formula of ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Brimonidine tartrate ophthalmic solution 0.2% is a relatively selective alpha-2 adrenergic receptor agonist with a peak ocular hypotensive effect occurring at two ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively ...
-
14 CLINICAL STUDIES
Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Brimonidine tartrate has ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Brimonidine tartrate ophthalmic solution 0.2% is supplied sterile, in white opaque plastic dropper bottles as follows: 5 mL - NDC 17478-715-10 - 10 mL - NDC 17478-715-11 - 15 mL - NDC ...
-
17 PATIENT COUNSELING INFORMATION
Handling the Container - Instruct patients that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Container Label: NDC 17478-715-10 - 5 mL - Brimonidine - Tartrate - Ophthalmic - Solution, 0.2% Rx only Sterile
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Carton Label: NDC 17478-715-10 - Brimonidine - Tartrate - Ophthalmic - Solution, 0.2% Rx Only - 5 mL Sterile - Akorn Logo
-
INGREDIENTS AND APPEARANCEProduct Information