Label: GENTAMICIN SULFATE solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 17478-283-10 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONSterile - Rx only
-
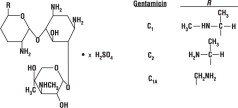
DESCRIPTIONGentamicin sulfate is a water-soluble antibiotic of the aminoglycoside group. Gentamicin Sulfate Ophthalmic Solution is a sterile, aqueous solution for ophthalmic use. Each mL contains: Active ...
-
CLINICAL PHARMACOLOGYMicrobiology - Gentamicin sulfate is active in vitro against many strains of the following microorganisms: Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes ...
-
INDICATIONS AND USAGEGentamicin Sulfate Sterile Ophthalmic Solution is indicated in the topical treatment of ocular bacterial infections, including conjunctivitis, keratitis, keratoconjunctivitis, corneal ulcers ...
-
CONTRAINDICATIONSGentamicin Sulfate Sterile Ophthalmic Solution is contraindicated in patients with known hypersensitivity to any of the components.
-
WARNINGSNOT FOR INJECTION INTO THE EYE. Gentamicin Sulfate Ophthalmic Solution is not for injection. It should never be injected subconjunctivally, nor should it be directly introduced into the anterior ...
-
PRECAUTIONSGeneral - Prolonged use of topical antibiotics may give rise to overgrowth of nonsusceptible organisms including fungi. Bacterial resistance to gentamicin may also develop. If purulent discharge ...
-
ADVERSE REACTIONSBacterial and fungal corneal ulcers have developed during treatment with gentamicin ophthalmic preparations. The most frequently reported adverse reactions are ocular burning and irritation upon ...
-
DOSAGE AND ADMINISTRATIONGentamicin Sulfate sterile ophthalmic solution; Instill one or two drops into the affected eye(s) every four hours. In severe infections, dosage may be increased to as much as two drops once every ...
-
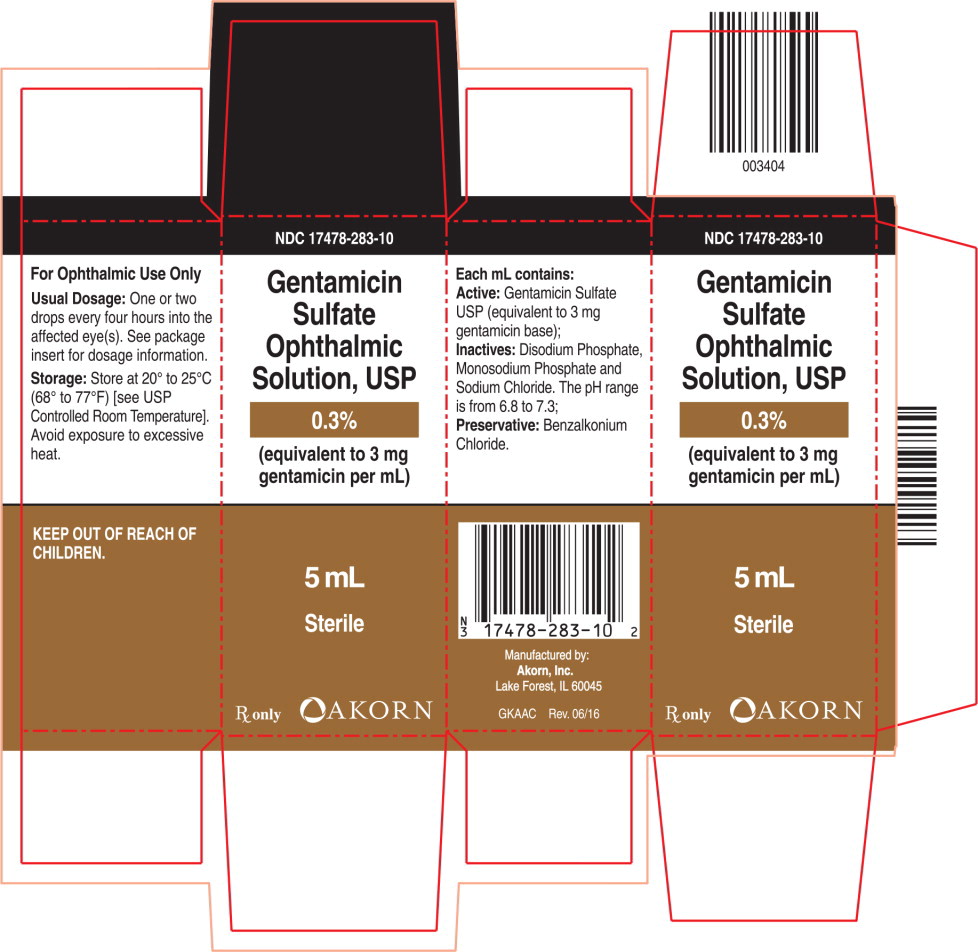
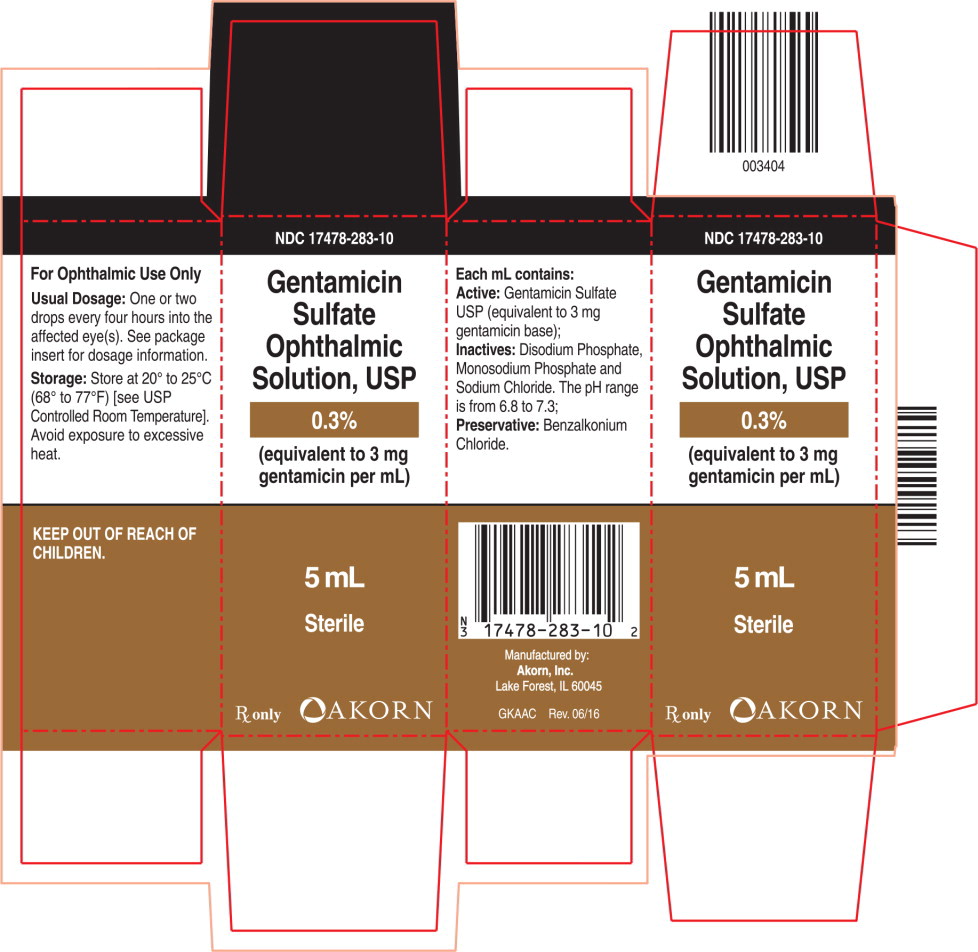
HOW SUPPLIEDGentamicin Sulfate ophthalmic solution - Sterile, 5-mL plastic dropper bottle, box of one. (NDC 17478-283-10) STORAGE: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room ...
-
SPL UNCLASSIFIED SECTIONAkorn - Manufactured by: Akorn, Inc. Lake Forest, IL 60045 - GK00N - Rev. 06/16
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Container Label: NDC 17478-283-10 - Gentamicin - Sulfate Ophthalmic - Solution, USP - 0.3% (equivalent to 3 mg - gentamicin per mL) Sterile - Rx only 5 mL
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Carton Label: NDC 17478-283-10 - Gentamicin - Sulfate - Ophthalmic - Solution, USP - 0.3% (equivalent to 3 mg - gentamicin per mL) 5 mL - Sterile - Rx only Akorn Logo
-
INGREDIENTS AND APPEARANCEProduct Information