Label: ATROPINE- atropine sulfate solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 17478-215-02, 17478-215-05, 17478-215-15 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ATROPINE SULFATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for ATROPINE SULFATE OPHTHALMIC ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGE
Atropine Sulfate Ophthalmic Solution, USP 1% is indicated for: 1.1 Cycloplegia - 1.2 Mydriasis - 1.3 Penalization of the healthy eye in the treatment of amblyopia
-
2. DOSAGE AND ADMINISTRATION
In individuals from three (3) months of age or greater, 1 drop topically to the cul-de-sac of the conjunctiva, forty minutes prior to the intended maximal dilation time. In individuals 3 ...
-
3. DOSAGE FORMS AND STRENGTHS
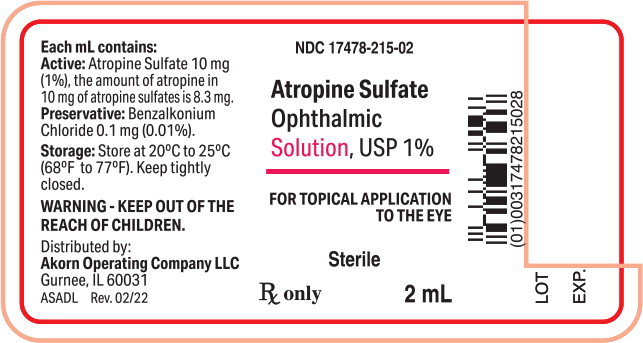
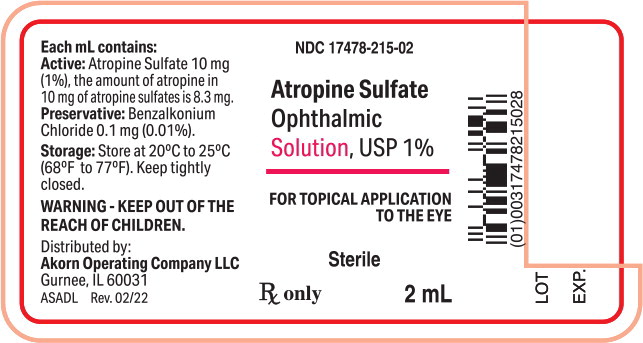
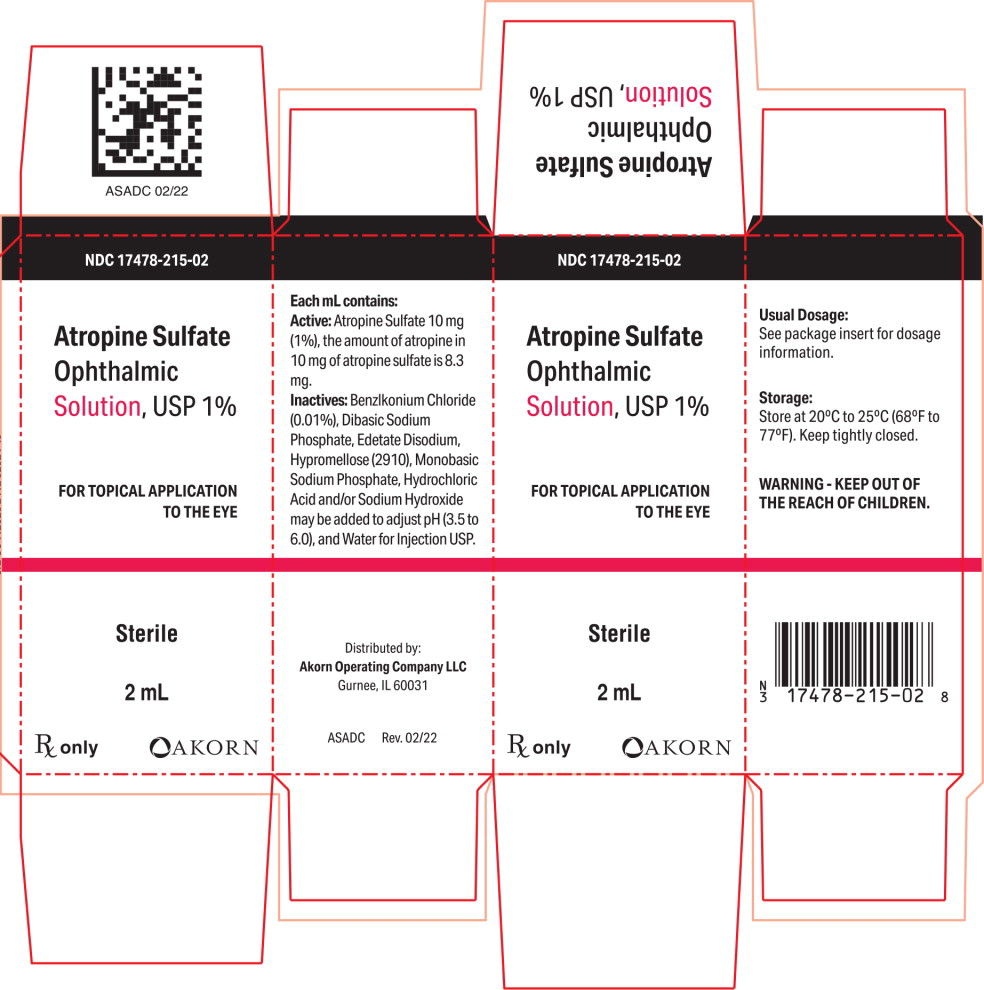
Atropine Sulfate Ophthalmic Solution, USP 1%: each mL contains 10 mg of atropine sulfate equivalent to 8.3 mg of atropine.
-
4. CONTRAINDICATIONS
4.1 Hypersensitivity to any Component of this Medication - Atropine sulfate ophthalmic solution should not be used in anyone who has demonstrated a previous hypersensitivity or known allergic ...
-
5. WARNINGS AND PRECAUTIONS
5.1 Photophobia and Blurred Vision - Photophobia and blurred vision due to pupil unresponsiveness and cycloplegia may last up to 2 weeks. 5.2 Elevation of Blood Pressure - Elevation in blood ...
-
6. ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling: Photophobia and Blurred Vision [See Warnings and Precautions (5.1)] Elevation in Blood Pressure [See ...
-
7. DRUG INTERACTIONS
7.1 Monoamine oxidase inhibitors (MAOI) The use of atropine and monoamine oxidase inhibitors (MAOI) is generally not recommended because of the potential to precipitate hypertensive ...
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Category C: There are no adequate and well-controlled studies of atropine sulfate in pregnant women. Animal development and reproduction studies have not been ...
-
10. OVERDOSAGE
In the event of accidental ingestion or toxic overdosage with atropine sulfate ophthalmic solution, supportive care may include a short acting barbiturate or diazepam as needed to control marked ...
-
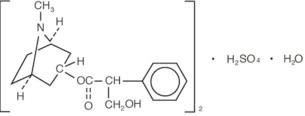
11. DESCRIPTION
Atropine Sulfate Ophthalmic Solution, USP 1% is a sterile topical anticholinergic for ophthalmic use. The active ingredient is represented by the chemical structure: Chemical Name ...
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Atropine is a reversible antagonist of muscarine-like actions of acetyl-choline and is therefore classified as an antimuscarinic agent. Atropine is relatively ...
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Atropine sulfate was negative in the salmonella/microsome mutagenicity test. Studies to evaluate carcinogenicity and impairment of ...
-
14. CLINICAL STUDIES
Topical administration of atropine sulfate ophthalmic solution 1% results in cycloplegia and mydriasis which has been demonstrated in several controlled clinical studies in adults and pediatric ...
-
16. HOW SUPPLIED/STORAGE AND HANDLING
Atropine Sulfate Ophthalmic Solution, USP 1% is supplied in a plastic dropper bottle with a red cap in the following sizes: NDC 17478-215-02 2 mL fill in 6cc bottle - NDC ...
-
17. PATIENT COUNSELING INFORMATION
Advise patients not to touch the dropper tip to any surface as this may contaminate the solution. Advise patients that drops will sting upon instillation and advise patients that they will ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Container Label: NDC 17478-215-02 - Atropine Sulfate - Ophthalmic - Solution, USP 1% FOR TOPICAL APPLICATION - THE EYE - Sterile - Rx only 2 mL
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for Carton Label: NDC 17478-215-02 - Atropine Sulfate - Ophthalmic - Solution, USP 1% FOR TOPICAL APPLICATION - TO THE EYE - Sterile - 2 mL - Rx only Akorn Logo
-
INGREDIENTS AND APPEARANCEProduct Information