Label: GEMCITABINE injection, solution
- NDC Code(s): 16729-391-30, 16729-419-03, 16729-423-33, 16729-426-05
- Packager: Accord Healthcare Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GEMCITABINE INJECTION safely and effectively. See full prescribing information for GEMCITABINE INJECTION. GEMCITABINE ...
-
Table of ContentsTable of Contents
-
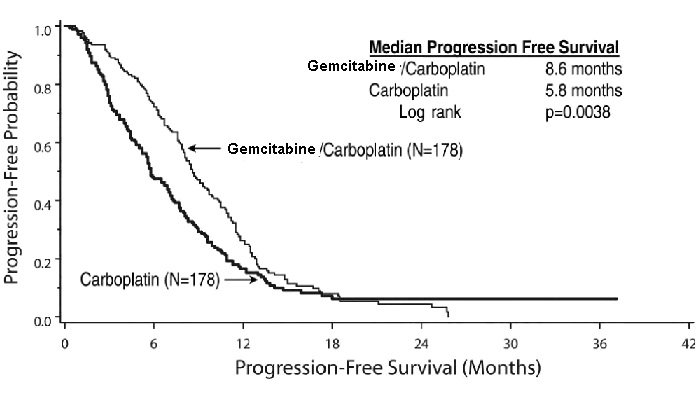
1 INDICATIONS AND USAGE1.1 Ovarian Cancer - Gemcitabine Injection in combination with carboplatin is indicated for the treatment of patients with advanced ovarian cancer that has relapsed at least 6 months after ...
-
2 DOSAGE AND ADMINISTRATION2.1 Ovarian Cancer - Recommended Dose and Schedule - The recommended dosage of Gemcitabine Injection is 1000 mg/m - 2intravenously over 30 minutes on Days 1 and 8 of each 21-day cycle in ...
-
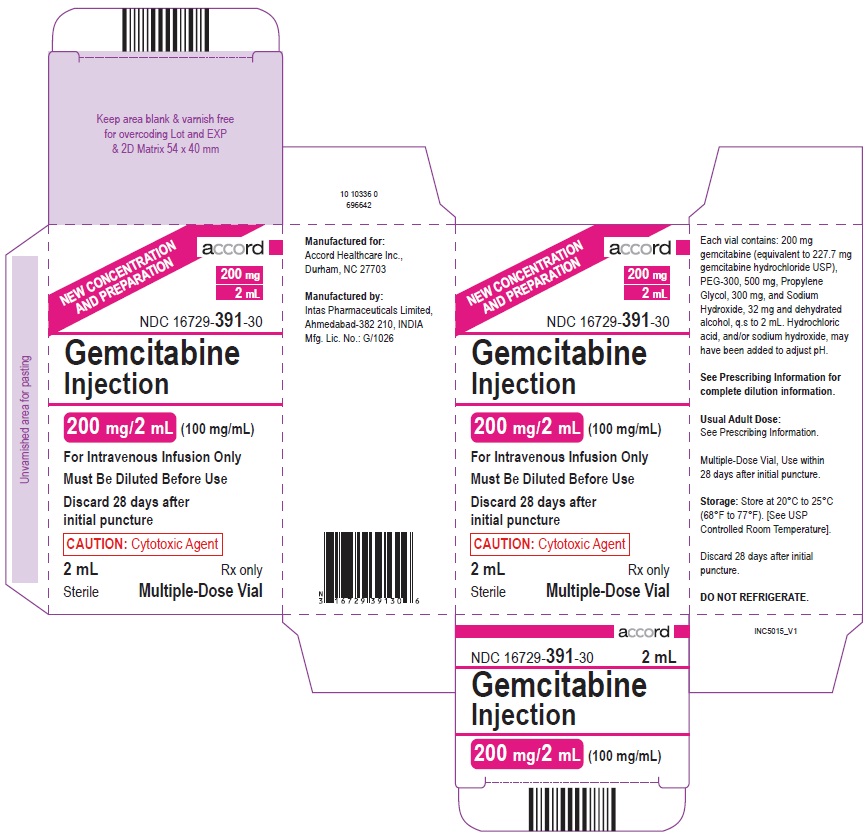
3 DOSAGE FORMS AND STRENGTHSInjection: 100 mg/mL of gemcitabine as a clear, colorless to pale yellow solution in sterile multiple-dose vials: 200 mg/2 mL (100 mg/mL) 1 g/10 mL (100 mg/mL) 1.5 g/15 mL (100 mg/mL) 2 g/20 mL ...
-
4 CONTRAINDICATIONSGemcitabine Injection is contraindicated in patients with a known hypersensitivity to gemcitabine. Reactions include anaphylaxis - [see Adverse Reactions ( 6.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Schedule-Dependent Toxicity - In clinical trials evaluating the maximum tolerated dose of gemcitabine, prolongation of the infusion time beyond 60 minutes or more frequent than weekly dosing ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hypersensitivity - [see Contraindications ( 4)] Schedule-Dependent Toxicity ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on animal data and its mechanism of action, Gemcitabine Injection can cause fetal harm when administered to a pregnant woman - [see Clinical Pharmacology ...
-
10 OVERDOSAGEThere is no known antidote for overdoses of gemcitabine. Myelosuppression, paresthesias, and severe rash were the principal toxicities seen when a single dose as high as 5700 mg/m - 2was ...
-
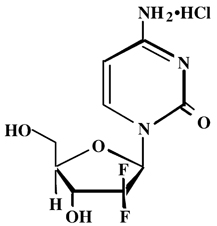
11 DESCRIPTIONGemcitabine is a nucleoside metabolic inhibitor. Gemcitabine hydrochloride is 2’-deoxy-2’,2’-difluorocytidine monohydrochloride (β-isomer) with the following molecular structure:. Gemcitabine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Gemcitabine kills cells undergoing DNA synthesis and blocks the progression of cells through the G1/S-phase boundary. Gemcitabine is metabolized by nucleoside kinases ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies to evaluate the carcinogenic potential of gemcitabine have not been conducted. Gemcitabine was mutagenic in an ...
-
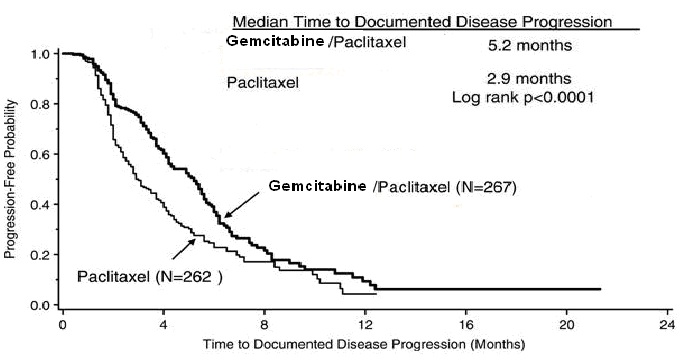
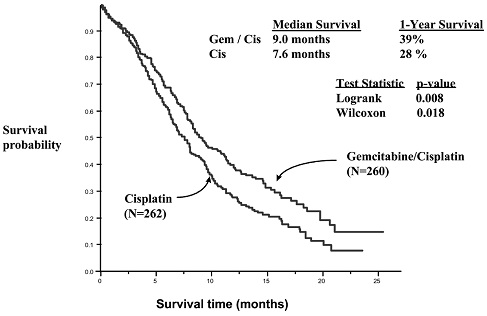
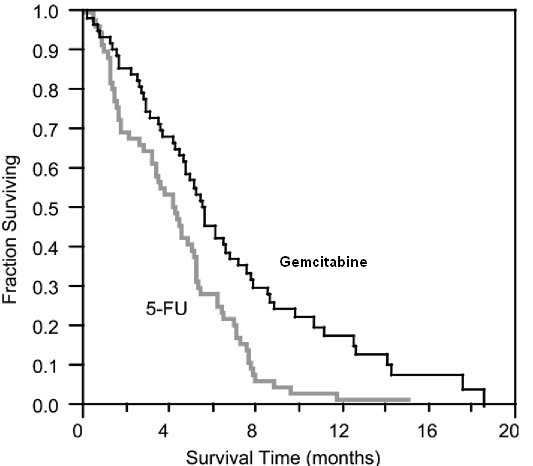
14 CLINICAL STUDIES14.1 Ovarian Cancer - The efficacy of gemcitabine was evaluated in a randomized trial (Study 1) conducted in women with advanced ovarian cancer that had relapsed at least 6 months after ...
-
15 REFERENCES1.“OSHA Hazardous Drugs. ”OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGemcitabine Injection is a clear colorless to pale yellow solution available in sterile multiple-dose vials containing: VialNDC number - 200 mg/2 mL (100 mg/mL) 1 g/10 mL (100 mg/mL ...
-
17 PATIENT COUNSELING INFORMATIONMyelosuppression - Advise patients of the risks of myelosuppression. Instruct patients to immediately contact their healthcare provider should any signs or symptoms of infection, including fever ...
-
SPL UNCLASSIFIED SECTIONManufactured For: Accord Healthcare, Inc., 8041 Arco Corporate Drive, Suite 200, Raleigh, NC 27617, USA. Manufactured By: Intas Pharmaceuticals Limited ...
-
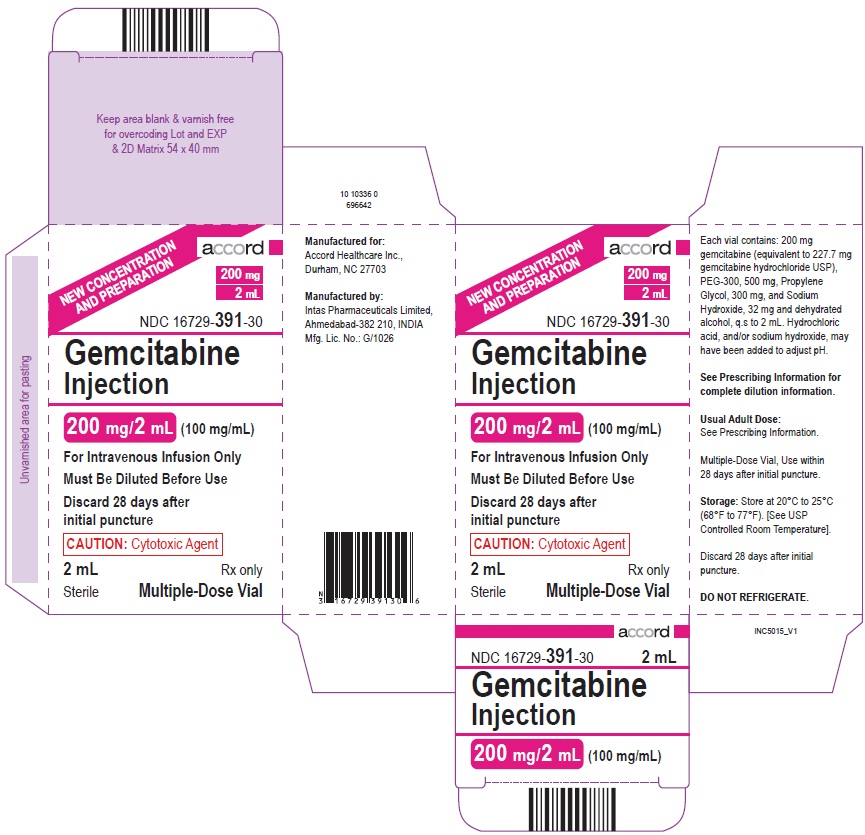
PRINCIPAL DISPLAY PANELPACKAGE CARTON – 200 mg/2 mL
-
PRINCIPAL DISPLAY PANELPACKAGE CONTAINER – 200 mg/2 mL
-
PRINCIPAL DISPLAY PANELPACKAGE CARTON – 1 g/10 mL
-
PRINCIPAL DISPLAY PANELPACKAGE CONTAINER – 1 g/10 mL
-
PRINCIPAL DISPLAY PANELPACKAGE CARTON – 1.5 g/15 mL
-
PRINCIPAL DISPLAY PANELPACKAGE CONTAINER – 1.5 g/15 mL
-
PRINCIPAL DISPLAY PANELPACKAGE CARTON – 2 g/20 mL
-
PRINCIPAL DISPLAY PANELPACKAGE CONTAINER – 2 g/20 mL
-
INGREDIENTS AND APPEARANCEProduct Information