Label: EPLERENONE tablet, film coated
- NDC Code(s): 16729-293-10, 16729-293-15, 16729-293-16, 16729-293-17, view more
- Packager: Accord Healthcare, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EPLERENONE TABLETS safely and effectively. See full prescribing information for EPLERENONE TABLETS. EPLERENONE tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.2 Hypertension - Eplerenone tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular (CV ...
-
2 DOSAGE AND ADMINISTRATION2.2 Hypertension - The recommended starting dose of eplerenone tablets are 50 mg administered once daily. The full therapeutic effect of eplerenone tablet is apparent within 4 weeks. For patients ...
-
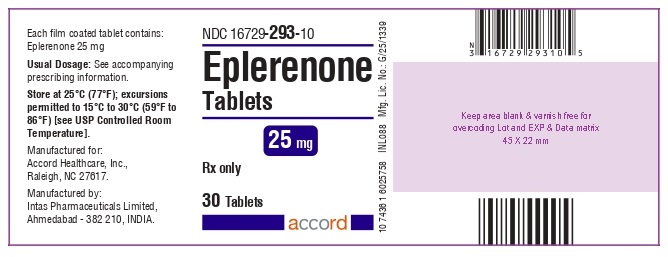
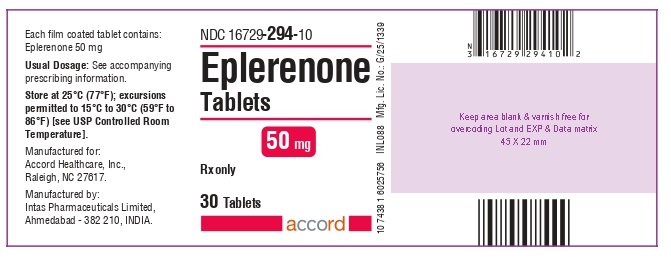
3 DOSAGE FORMS AND STRENGTHS25 mg tablets: yellow diamond shape biconvex film-coated tablets debossed with - E1on one side and plain on the other side. 50 mg tablets: yellow diamond shape biconvex film-coated ...
-
4 CONTRAINDICATIONSFor All Patients - Eplerenone tablets are contraindicated in all patients with: serum potassium >5.5 mEq/L at initiation, creatinine clearance ≤30 mL/min, or - concomitant administration of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hyperkalemia - The risk of hyperkalemia is higher in patients with impaired renal function, proteinuria , diabetes and those concomitantly treated with ARBs, NSAIDs and moderate CYP3A ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Hyperkalemia - [See - Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 CYP3A Inhibitors - Eplerenone metabolism is predominantly mediated via CYP3A. Do not use eplerenone with drugs that are strong inhibitors of CYP3A. [See - Contraindications (4)and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data from published case reports on eplerenone use during pregnancy are insufficient to establish a drug-associated risk of major birth defects ...
-
10 OVERDOSAGENo cases of human overdosage with eplerenone have been reported. Lethality was not observed in mice, rats, or dogs after single oral doses that provided C - maxexposures at least 25 times higher ...
-
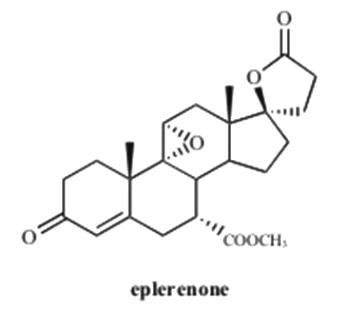
11 DESCRIPTIONEplerenone tablet contains eplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor. Eplerenone is chemically described as Pregn-4-ene-7,21-dicarboxylic acid ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Eplerenone binds to the mineralocorticoid receptor and blocks the binding of aldosterone, a component of the renin-angiotensin-aldosterone-system (RAAS). Aldosterone ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Eplerenone was non-genotoxic in a battery of assays including in vitro bacterial mutagenesis (Ames test in - Salmonellaspp. and ...
-
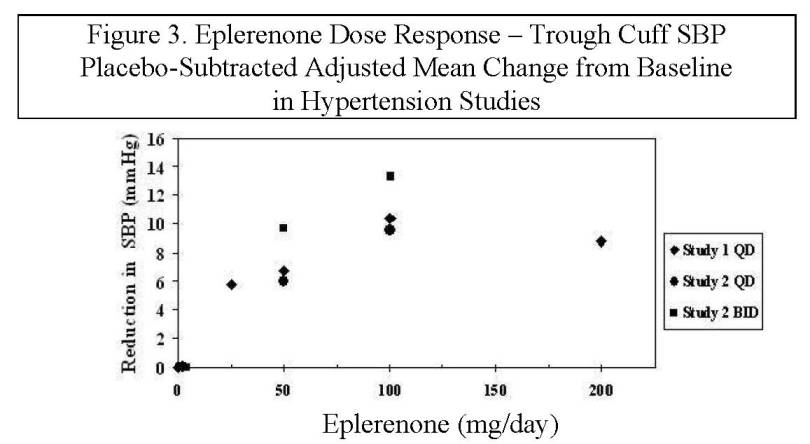
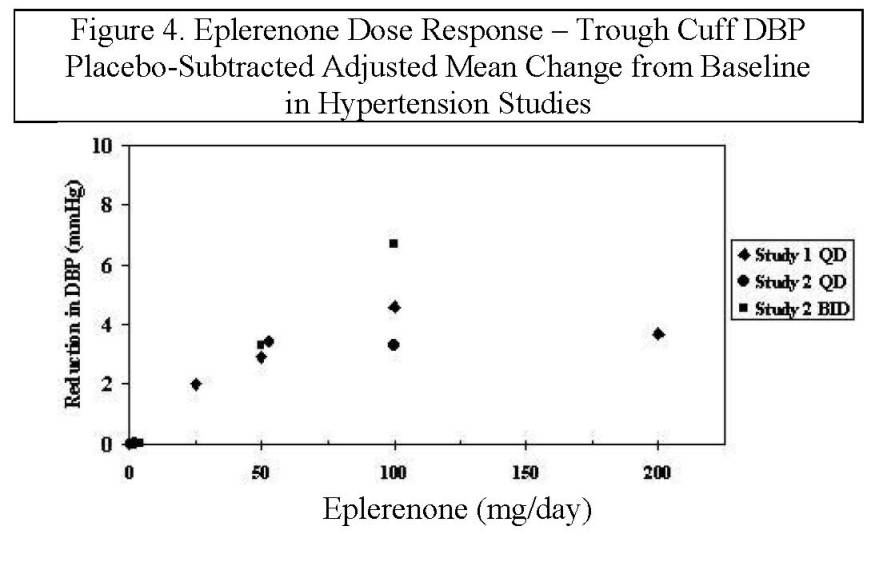
14 CLINICAL STUDIES14.2 Hypertension - The safety and efficacy of eplerenone has been evaluated in clinical studies of 3091 hypertensive patients. The studies included 46% women, 14% Blacks, and 22% elderly (age ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEplerenone tablets, 25 mg, are yellow diamond shape biconvex film-coated tablets, debossed with “E1” on one side and plain on the other side. They are supplied as follows: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients receiving eplerenone tablets: Not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician. [See - Warnings ...
-
SPL UNCLASSIFIED SECTIONManufactured For: Accord Healthcare, Inc., 8041 Arco Corporate Drive, Suite 200 - Raleigh, NC 27617, USA. Manufactured By: Intas Pharmaceuticals Limited, Plot ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 25 mg Tablet - Bottle of 30
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 50 mg Tablet - Bottle of 30
-
INGREDIENTS AND APPEARANCEProduct Information