Label: GLIPIZIDE tablet, extended release
- NDC Code(s): 16714-894-01, 16714-895-01, 16714-895-02, 16714-896-01, view more
- Packager: Northstar Rx LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLIPIZIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for GLIPIZIDE EXTENDED-RELEASE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGlipizide extended-release tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. 1.1 Limitations of Use - Glipizide ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - Glipizide extended-release tablets should be administered orally with breakfast or the first main meal of the day. The recommended starting dose of glipizide ...
-
3 DOSAGE FORMS AND STRENGTHSGlipizide extended-release tablets, 2.5 mg are yellow colored, round, biconvex film-coated tablets imprinted with "2" on one side with black ink and plain on the other side. Glipizide ...
-
4 CONTRAINDICATIONSGlipizide is contraindicated in patients with: Known hypersensitivity to glipizide or any of the product's ingredients. Hypersensitivity to sulfonamide derivatives.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia - All sulfonylurea drugs, including glipizide extended-release tablets, are capable of producing severe hypoglycemia [see Adverse Reactions (6)]. Concomitant use of glipizide ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in more detail below and elsewhere in the labeling: Hypoglycemia [see Warnings and Precautions (5.1) ] Hemolytic anemia [see ...
-
7 DRUG INTERACTIONS7.1 Drugs Affecting Glucose Metabolism - A number of medications affect glucose metabolism and may require glipizide extended-release tablets dose adjustment and close monitoring for ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from a small number of published studies and postmarketing experience with glipizide extended-release tablets use in pregnancy over decades have not ...
-
10 OVERDOSAGEOverdosage of sulfonylureas including glipizide extended-release tablets can produce severe hypoglycemia. Mild hypoglycemic symptoms without loss of consciousness or neurologic findings should be ...
-
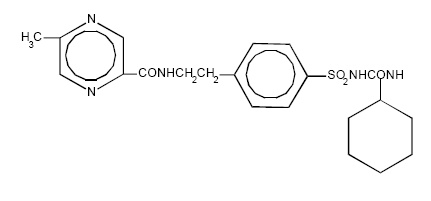
11 DESCRIPTIONGlipizide extended-release tablets are an oral sulfonylurea. The Chemical Abstracts name of glipizide is 1-cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido) ethyl] phenyl]sulfonyl]urea. The ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Glipizide primarily lowers blood glucose by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A twenty month study in rats and an eighteen month study in mice at doses up to 75 times the maximum human dose revealed no evidence ...
-
15 REFERENCES1. Diabetes, 19, SUPP. 2: 747–830, 1970
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGlipizide Extended-release Tablets, 2.5 mg are yellow colored, round, biconvex film-coated tablets imprinted with "2" on one side with black ink and plain on the other side and are supplied as ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Inform patients of the potential adverse reactions of glipizide extended-release tablets including ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Northstar Rx LLC - Memphis, TN 38141. Manufactured by: Zydus Lifesciences Ltd. Ahmedabad, India. Rev.: 06/22
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Glipizide (GLIP-i-zide) Extended-release Tablets - The 2.5 mg tablets contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial ...
-
PATIENT PACKAGE INSERTManufactured for: Northstar Rx LLC - Memphis, TN 38141. Manufactured by: Zydus Lifesciences Ltd. Ahmedabad, India. Rev.: 06/22
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 16714-894-01 in bottle of 30 tablets - Glipizide Extended-release Tablets, 2.5 mg - Rx only - 30 tablets - NDC 16714-895-01 in bottle of 100 tablets - Glipizide Extended-release Tablets, 5 mg - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information