Label: DESONIDE cream
- NDC Code(s): 16714-729-01, 16714-729-02
- Packager: NORTHSTAR RX LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Dermatologic Use Only - Not For Ophthalmic Use - Rx Only
-
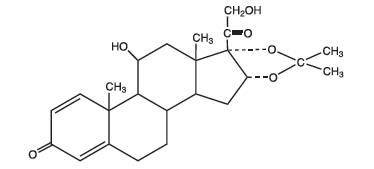
DESCRIPTIONDesonide Cream, 0.05% contains desonide (Pregna-1,4-diene-3,20-dione,11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-,(11β,16α-)) a synthetic corticosteroid for topical dermatologic use. The ...
-
CLINICAL PHARMACOLOGYLike other topical corticosteroids, desonide has anti-inflammatory, antipruritic and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in ...
-
INDICATIONS AND USAGEDesonide cream, 0.05% is a low potency corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. It should not be used for ...
-
CONTRAINDICATIONSDesonide cream, 0.05% is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
-
PRECAUTIONSGeneral - Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency ...
-
ADVERSE REACTIONSIn controlled clinical trials, the total incidence of adverse reactions associated with the use of desonide cream, 0.05% was approximately 1%. The adverse reactions for desonide cream, 0.05% were ...
-
OVERDOSAGETopically applied desonide cream, 0.05% can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
-
DOSAGE AND ADMINISTRATIONDesonide cream, 0.05% should be applied to the affected area as a thin film two to four times daily depending on the severity of the condition. As with other corticosteroids, therapy should be ...
-
HOW SUPPLIEDDesonide Cream, 0.05% is supplied in 15 g (NDC 16714-729-01) tubes. Desonide Cream, 0.05% is supplied in 60 g (NDC 16714-729-02) tubes. STORAGE - Store at 20° to 25°C (68° to 77°F) [see USP ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Northstar RxLLC, Memphis, TN 38141 - Manufactured by: Taro Pharmaceutical Industries Ltd. Toll Free: 1-800-206-7821 - Haifa Bay, Israel 2624761 - Product of Italy - Revised: June ...
-
PRINCIPAL DISPLAY PANEL - 60 g Tube CartonRx only NDC 16714-729-02 - Desonide Cream 0.05% FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. NORTHSTARX® 60 g
-
INGREDIENTS AND APPEARANCEProduct Information