Label: IPRATROPIUM BROMIDE spray, metered
- NDC Code(s): 16714-527-01
- Packager: NORTHSTAR RX LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ATTENTION PHARMACIST: Detach "Patient's Instructions for Use" from package insert and dispense with the product.
-
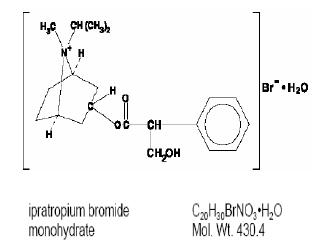
DESCRIPTIONThe active ingredient in ipratropium bromide nasal solution is ipratropium bromide monohydrate. It is an anticholinergic agent chemically described as 8-azoniabicyclo (3.2.1) octane ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action ...
-
INDICATIONS AND USAGEIpratropium bromide nasal solution, 0.06% is indicated for the symptomatic relief of rhinorrhea associated with the common cold or seasonal allergic rhinitis for adults and children age 5 years ...
-
CONTRAINDICATIONSIpratropium bromide nasal solution, 0.06% is contraindicated in patients with a history of hypersensitivity to atropine or its derivatives, or to any of the other ingredients.
-
WARNINGSImmediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema. If ...
-
PRECAUTIONSGeneral - Effects Seen with Anticholinergic Drugs: Ipratropium bromide nasal solution, 0.06% should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia, or ...
-
ADVERSE REACTIONSAdverse reaction information on ipratropium bromide nasal solution, 0.06% in patients with the common cold was derived from two multicenter, vehicle-controlled clinical trials involving 1,276 ...
-
OVERDOSAGEAcute overdosage by intranasal administration is unlikely since ipratropium bromide is not well absorbed systemically after intranasal or oral administration. Following administration of a 20 mg ...
-
DOSAGE AND ADMINISTRATIONFor Symptomatic Relief of Rhinorrhea Associated with the Common Cold - The recommended dose of ipratropium bromide nasal solution, 0.06% is two sprays (84 mcg) per nostril three or four times ...
-
HOW SUPPLIEDIpratropium Bromide Nasal Solution, 0.06% is supplied in a white high density polyethylene (HDPE) bottle fitted with a metered nasal spray pump, a green safety clip to prevent accidental discharge ...
-
Patients Instructions for UseIpratropium Bromide Nasal Solution, 0.06% Nasal spray - (ip" ra troe' pee um broe' mide) Rx Only - Read complete instructions carefully before using. In order to ensure proper dosing, do ...
-
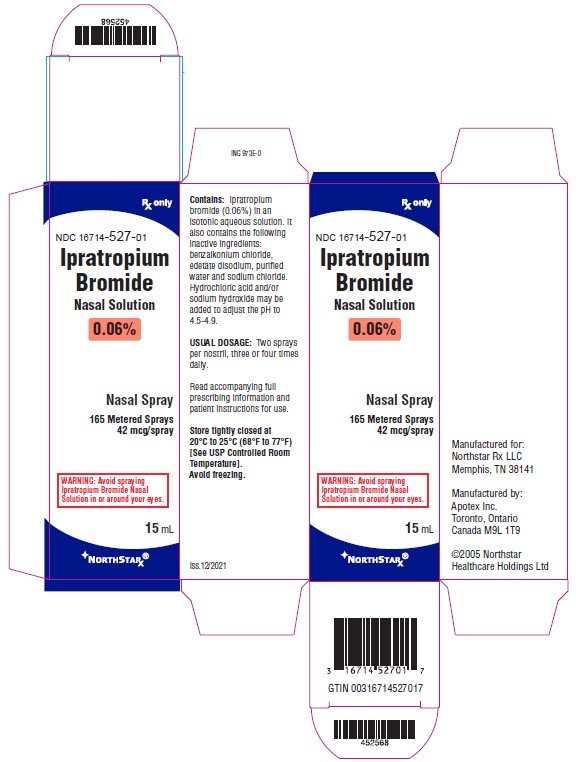
PRINCIPAL DISPLAY PANEL- 0.06% Bottle Label
Northstar Rx LLC NDC 16714-527-01 - Ipratropium Bromide Nasal Solution - 0.06% Nasal Spray - 165 Metered Sprays - 42 mcg/spray - Rx Only - 15 mL
-
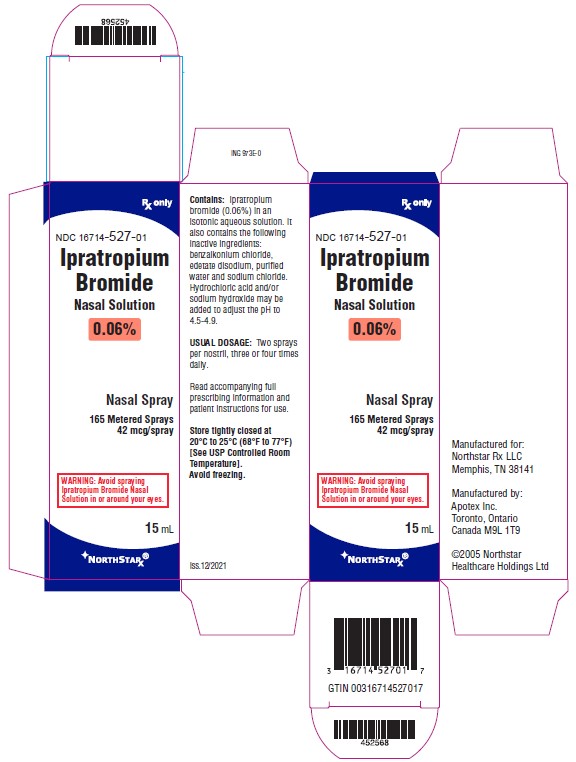
PRINCIPAL DISPLAY PANEL- 0.06% Carton Label
Northstar Rx LLC NDC 16714-527-01 - Ipratropium Bromide Nasal Solution - 0.06% Nasal Spray - 165 Metered Sprays - 42 mcg/spray - Rx Only - 15 mL
-
INGREDIENTS AND APPEARANCEProduct Information