Label: FEBUXOSTAT tablet, film coated
- NDC Code(s): 16714-059-01, 16714-060-01
- Packager: NorthStar RxLLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FEBUXOSTAT TABLETS safely and effectively. See full prescribing information for FEBUXOSTAT TABLETS. FEBUXOSTAT tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR DEATH
Close
Gout patients with established cardiovascular (CV) disease treated with febuxostat tablets had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study [see Warnings and Precautions (5.1)].
Consider the risks and benefits of febuxostat tablets when deciding to prescribe or continue patients on febuxostat tablets. Febuxostat tablets should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable [see Indications and Usage (1)]. -
1 INDICATIONS AND USAGEFebuxostat tablets are xanthine oxidase (XO) inhibitor indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended febuxostat tablets dosage is 40 mg or 80 mg once daily. The recommended starting dosage of febuxostat tablets is 40 mg once daily. For patients who do not ...
-
3 DOSAGE FORMS AND STRENGTHS40 mg tablets, round, biconvex, green colored, film-coated tablets with ‘721’ debossed on one side and plain on other side. 80 mg tablets, oval, biconvex, green colored, film-coated tablets with ...
-
4 CONTRAINDICATIONSFebuxostat tablets are contraindicated in patients being treated with azathioprine or mercaptopurine [see Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Death - In a cardiovascular (CV) outcome study, gout patients with established CV disease treated with febuxostat tablets had a higher rate of CV death compared to those ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the prescribing information: Cardiovascular Death [see Warnings and Precautions (5.1)] Hepatic Effects [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Xanthine Oxidase Substrate Drugs - Febuxostat is an XO inhibitor. Based on a drug interaction study in healthy patients, febuxostat altered the metabolism of theophylline (a substrate of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with febuxostat use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. No adverse ...
-
10 OVERDOSAGEFebuxostat was studied in healthy patients in doses up to 300 mg daily for seven days without evidence of dose-limiting toxicities. No overdose of febuxostat was reported in clinical studies ...
-
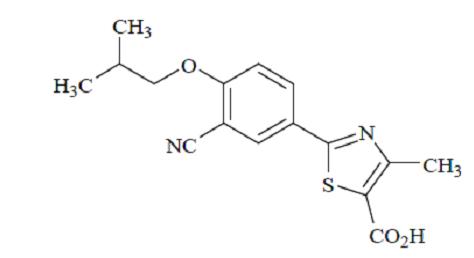
11 DESCRIPTIONFebuxostat is a xanthine oxidase inhibitor. The active ingredient in febuxostat tablets is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Febuxostat, a xanthine oxidase inhibitor, achieves its therapeutic effect by decreasing serum uric acid. Febuxostat is not expected to inhibit other enzymes involved in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two year carcinogenicity studies were conducted in F344 rats and B6C3F1 mice. Increased transitional cell papilloma and carcinoma of ...
-
14 CLINICAL STUDIESA serum uric acid level of less than 6 mg/dL is the goal of antihyperuricemic therapy and has been established as appropriate for the treatment of gout. 14.1 Management of Hyperuricemia in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFebuxostat 40 mg tablets are round, biconvex, green colored, film-coated tablets with ‘721’ debossed on one side and plain on other side and supplied as: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). CV Death - Inform patients that gout patients with established CV disease treated with febuxostat tablets had a ...
-
MEDICATION GUIDEMEDICATION GUIDE - Febuxostat (fe-BUX-oh-stat) tablets, for oral use - Read the Medication Guide that comes with febuxostat tablets before you start taking it and each time you get a ...
-
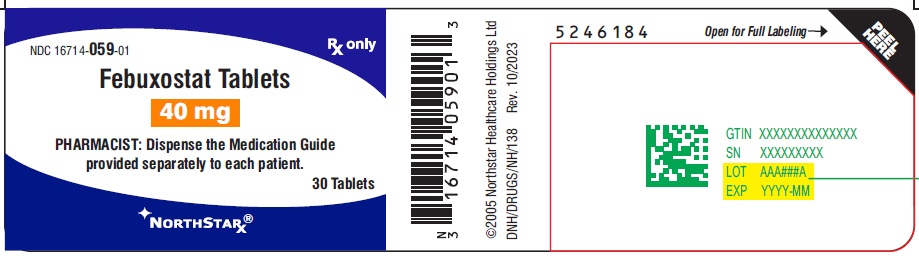
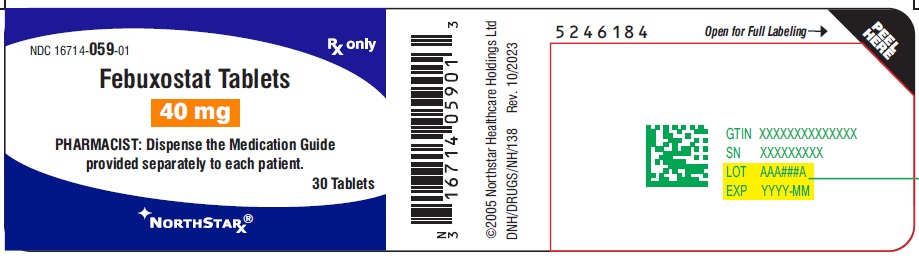
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-40mgNDC 16714-059-01 - Febuxostat Tablets - 40 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Rx only - 30 Tablets - NORTHSTAR
-
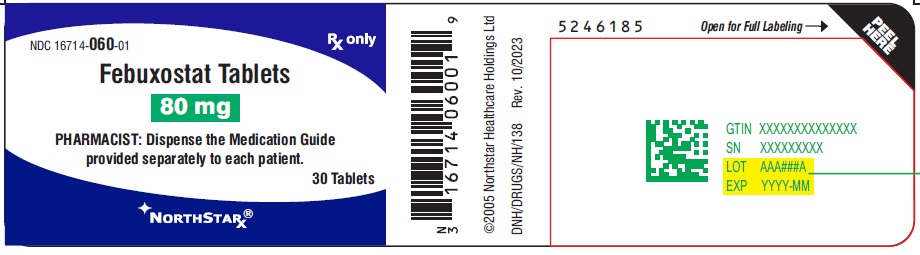
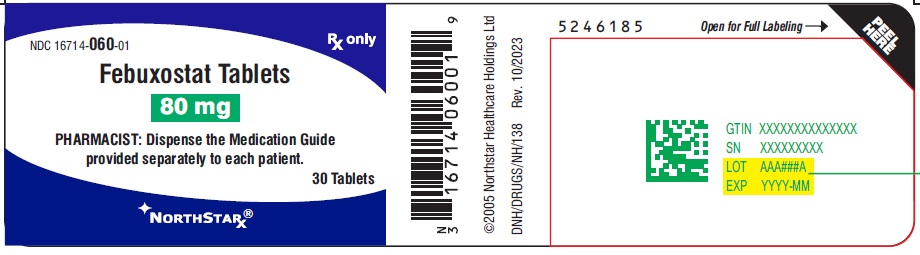
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-80mgNDC16714-060-01 - Febuxostat Tablets - 80 mg - PHARMACIST: Dispense the Medication Guide provided separately to each patient. Rx only - 30 Tablets - NORTHSTAR
-
INGREDIENTS AND APPEARANCEProduct Information