Label: MECLIZINE HYDROCHLORIDE tablet
MECLIZINE HYDROCHLORIDE tablet, chewable
- NDC Code(s): 16571-660-01, 16571-660-50, 16571-661-01, 16571-661-10, view more
- Packager: Rising Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated October 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MECLIZINE HYDROCHLORIDE safely and effectively. See full prescribing information for MECLIZINE HYDROCHLORIDE. MECLIZINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMeclizine hydrochloride is indicated for the treatment of vertigo associated with diseases affecting the vestibular system in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage is 25 mg to 100 mg daily administered orally, in divided doses, depending upon clinical response. 2.2 Administration ...
-
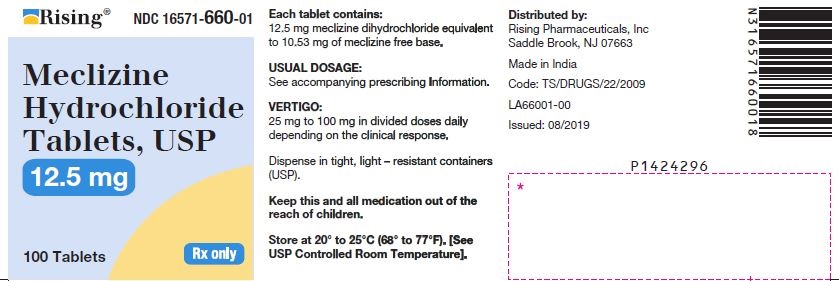
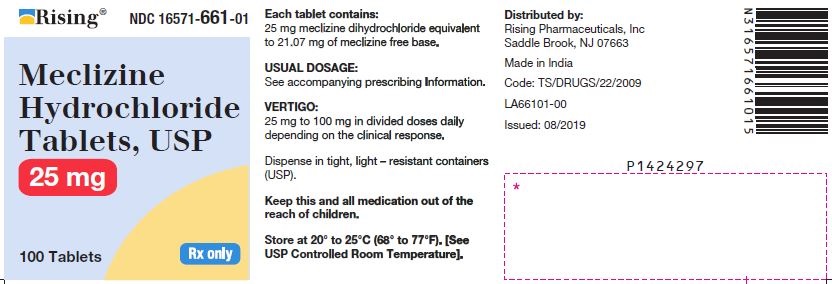
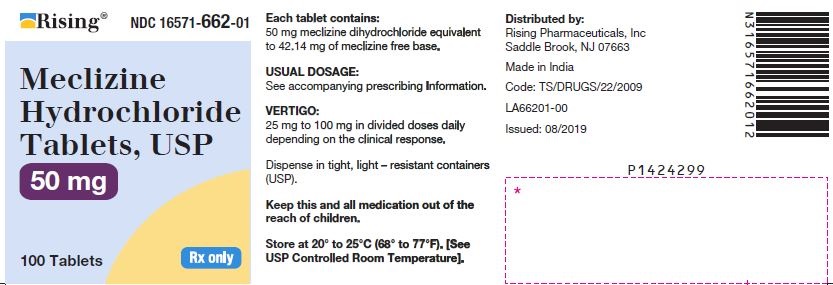
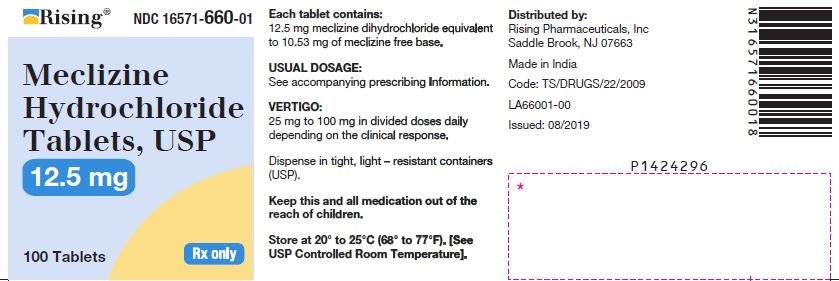
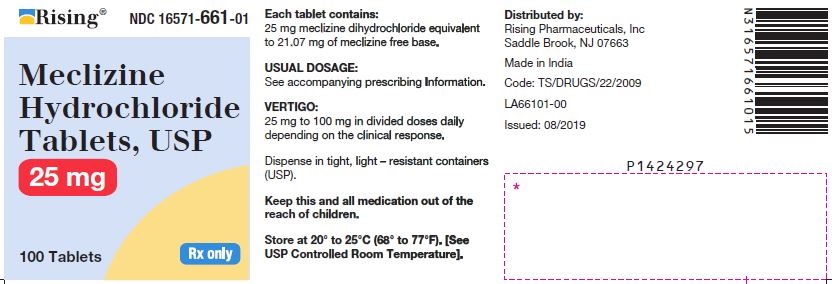
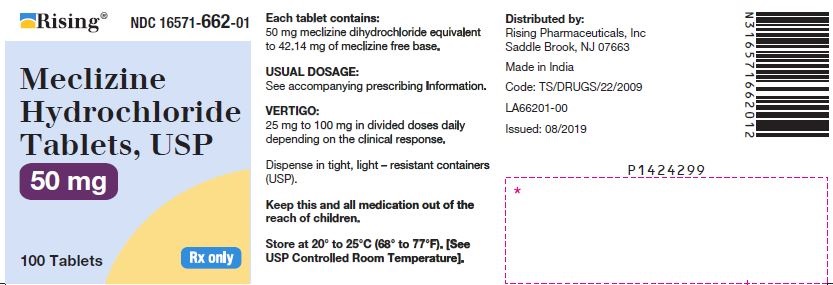
3 DOSAGE FORMS AND STRENGTHSTablets - 12.5 mg: oval-shaped, biconvex, two-layered tablet, one blue to pale blue layer debossed with “34” and one white to off white layer debossed with “L”. 25 mg: oval-shaped, biconvex ...
-
4 CONTRAINDICATIONSMeclizine hydrochloride is contraindicated in patients with a hypersensitivity to meclizine or any of the inactive ingredients [see Adverse Reactions (6) and Description (11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Drowsiness - Since drowsiness may occur with use of meclizine hydrochloride, patients should be warned of this possibility and cautioned against driving a car or operating dangerous ...
-
6 ADVERSE REACTIONSThe following adverse reactions associated with the use of meclizine hydrochloride were identified in clinical studies or postmarketing reports. Because some of these reactions were reported ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants - There may be increased CNS depression when meclizine hydrochloride is administered concurrently with other CNS depressants, including alcohol [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Data from epidemiological studies have not generally indicated a drug-associated risk of major birth defects with meclizine during pregnancy. However, in a ...
-
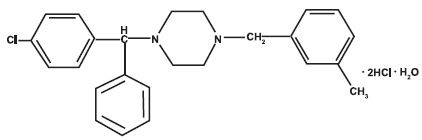
11 DESCRIPTIONMeclizine hydrochloride, a histamine (H1) receptor antagonist, is a white or slightly yellowish, crystalline powder. It has the following structural formula: Chemically, meclizine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which meclizine exerts its therapeutic effect is unknown but is presumed to involve antagonism of the histamine H1 receptor. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Animal studies to assess the carcinogenic potential of meclizine have not been conducted. Mutagenesis - Genetic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Tablets - Meclizine hydrochloride 12.5 mg tablets are oval shaped, biconvex, two-layered tablet, one blue to pale blue layer debossed with “34” and one white to off white ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Advise patients that the tablets must be swallowed whole, but chewable tablets must be chewed or crushed completely before swallowing [see Dosage and Administration ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 16571-660-01 - Meclizine Hydrochloride Tablets, USP - 12.5mg - Rx Only - Container Label - NDC 16571-661-01 - Meclizine Hydrochloride Tablets, USP - 25mg - Rx Only - Container ...
-
INGREDIENTS AND APPEARANCEProduct Information