Label: CHLORZOXAZONE tablet
- NDC Code(s): 16571-725-01, 16571-725-50, 16571-726-01, 16571-726-50, view more
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
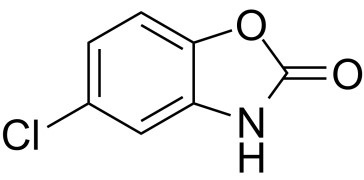
DESCRIPTIONFor Painful Musculoskeletal Conditions - PRESCRIBING INFORMATION - DESCRIPTION - Each 375 mg Chlorzoxazone tablet contains: Chlorzoxazone USP 375 mg. Each 500 mg Chlorzoxazone tablet ...
-
CLINICAL PHARMACOLOGYChlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the ...
-
INDICATIONS AND USAGEChlorzoxazone is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of ...
-
CONTRAINDICATIONSChlorzoxazone is contraindicated in patients with known intolerance to the drug.
-
WARNINGSSerious (including fatal) hepatocellular toxicity has been reported rarely in patients receiving chlorzoxazone. The mechanism is unknown but appears to be idiosyncratic and unpredictable. Factors ...
-
PRECAUTIONSChlorzoxazone should be used with caution in patients with known allergies or with a history of allergic reactions to drugs. If a sensitivity reaction occurs such as urticaria, redness, or itching ...
-
ADVERSE REACTIONSChlorzoxazone containing products are usually well tolerated. It is possible in rare instances that chlorzoxazone may have been associated with gastrointestinal bleeding. Drowsiness, dizziness ...
-
OVERDOSAGESymptoms: Initially, gastrointestinal disturbances such as nausea, vomiting, or diarrhea together with drowsiness, dizziness, lightheadedness or headache may occur. Early in the course there may ...
-
DOSAGE AND ADMINISTRATIONUsual Adult Dosage - Chlorzoxazone tablets 375 mg: One tablet three or four times daily. If adequate response is not obtained with this dose, the 375 mg tablets may be increased to two tablets ...
-
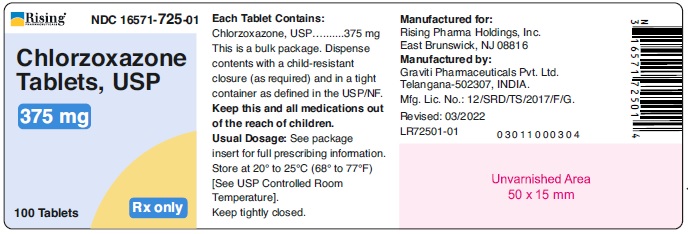
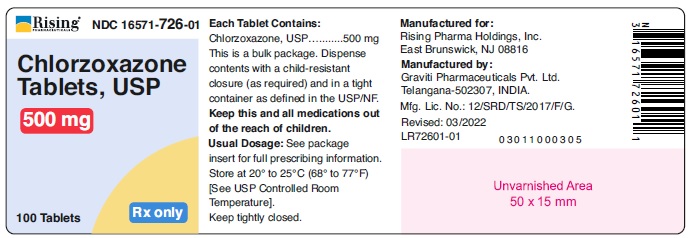
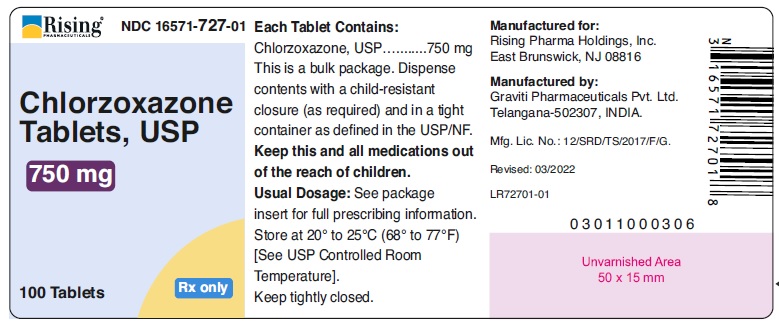
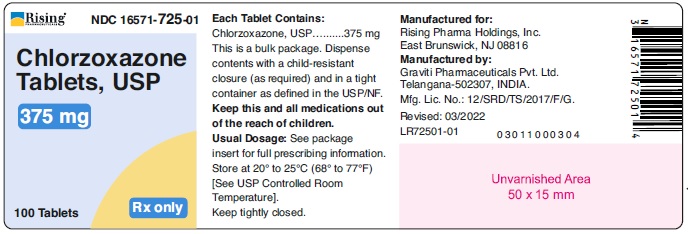
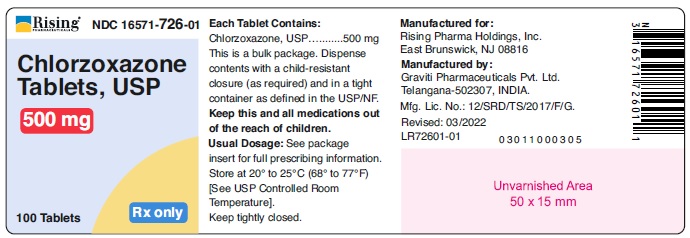
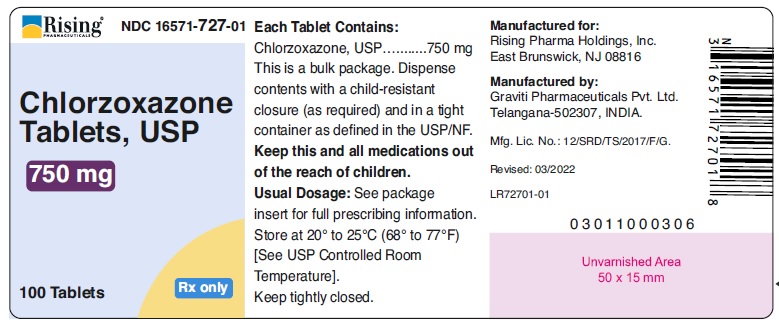
HOW SUPPLIEDChlorzoxazone tablets, USP are supplied as follows: 375 mg - White to off white capsule shaped tablet, debossed ‘126’ on one side and plain on other side. Bottle of 100 tablets with ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELRising® NDC 16571-725-01 - Chlorzoxazone - Tablets, USP - 375 mg - 100 Tablets Rx only - Rising® NDC 16571-726-01 - Chlorzoxazone - Tablets, USP - 500 mg - 100 Tablets Rx only ...
-
INGREDIENTS AND APPEARANCEProduct Information