Label: HYOSCYAMINE SULFATE tablet, extended release
- NDC Code(s): 16477-738-01
- Packager: Laser Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
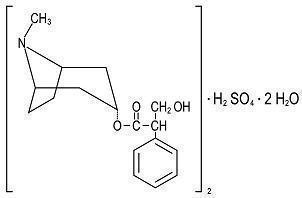
DESCRIPTIONEach extended-release tablet for oral administration contains: Hyoscyamine Sulfate, USP …… 0.375 mg - Hyoscyamine sulfate is one of the principal anticholinergic/ antispasmodic components of ...
-
INACTIVE INGREDIENTSInactive ingredients include: calcium phosphate dibasic, ethylcellulose, lactose monohydrate, magnesium stearate (veg.), microcrystalline cellulose, and stearic acid.
-
CLINICAL PHARMACOLOGYHyoscyamine has actions similar to those of atropine, but is more potent in both its central and peripheral effects. This product inhibits gastrointestinal propulsive motility and decreases ...
-
INDICATIONS AND USAGEThis product may be used in functional intestinal disorders to reduce symptoms such as those seen in mild dysenteries and diverticulitis. It can also be used to control gastric secretion, visceral ...
-
CONTRAINDICATIONSGlaucoma, obstructive uropathy, obstructive diseases of the gastrointestinal tract, paralytic ileum, intestinal atony of elderly or debilitated patients, unstable cardiovascular status, severe ...
-
WARNINGSHeat prostration can occur with drug use in the event of high environmental temperature. Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ...
-
PRECAUTIONSGeneral:Use caution in patients with hiatal hernia associated with reflex esophagitis. Use extreme caution and only when needed in patients with autonomic neuropathy, hyperthyroidism, coronary ...
-
ADVERSE REACTIONSNot all of the following adverse reactions have been reported with hyoscyamine sulfate. The following adverse reactions have been reported for pharmacologically similar drugs with ...
-
OVERDOSAGEThe signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot dry skin, dizziness, dryness of the mouth, difficulty in swallowing and CNS ...
-
DOSAGE AND ADMINISTRATIONUsual dosage: Adults and children over 12 years of age:1 or 2 tablets every 12 hours. Dosage may be adjusted to 1 tablet every 8 hours if needed. Do not exceed 4 tablets in 24 hours. Tablets ...
-
HOW SUPPLIEDHyoscyamine Sulfate ER Tablets are supplied as white, capsule shaped tablets debossed “738” on one side and plain on the opposite side. They are available in bottles of 100 tablets, NDC ...
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information