Label: SODIUM SULFACETAMIDE liquid
- NDC Code(s): 16477-410-06, 16477-410-12
- Packager: Laser Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Description:
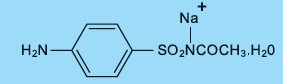
Each mL contains 100 mg of sodium sulfacetamide in a vehicle consisting of: citric acid, cocamidopropyl betaine, edetate disodium, glycerin, glyceryl stearate, methylparaben, PEG-6 caprylic/capric ...
-
CLINICAL PHARMACOLOGY:
Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide sensitive Gram-positive and Gram-negative microorganisms commonly isolated from secondary cutaneous pyogenic infections. It ...
-
INDICATIONS AND USAGE:
Sodium Sulfacetamide 10% Wash is intended for topical application in the following scaling dermatoses: seborrheic dermatitis and seborrhea sicca (dandruff). It also is indicated for the treatment ...
-
CONTRAINDICATIONS:
Sodium Sulfacetamide 10% Wash is contraindicated in persons with known or suspected hypersensitivity to sulfonamides or to any of the ingredients of the product.
-
WARNINGS:
Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has been reported following the use of sodium sulfacetamide topically. Cases ...
-
PRECAUTIONS:
For external use only. Not for ophthalmic use. General: Nonsusceptible organisms, including fungi, may proliferate with the use of this preparation. Hypersensitivity reactions may recur when ...
-
Information For Patients:
Patients should discontinue the use of Sodium Sulfacetamide 10% Wash if the condition becomes worse or if a rash develops in the area being treated or elsewhere. The use of Sodium Sulfacetamide ...
-
Drug Interactions:
Sodium Sulfacetamide 10% Wash is incompatible with silver preparations.
-
Pharmacology:
Sodium Sulfacetamide 10% Wash has a bacteriostatic effect against Gram-positive and Gram-negative miroorganisms commonly isolated from secondary cutaneous pyogenic infections.
-
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Long-term animal studies for carcinogenic potential have not been performed on Sodium Sulfacetamide 10% Wash to date. Studies on reprodution and fertility also have not been performed. Chromosomal ...
-
Pregnancy: Category C:
Animal reproduction studies have not been conducted with Sodium Sulfacetamide 10% Wash. It is also not known whether Sodium Sulfacetamide 10% Wash can affect reproduction capacity or cause fetal ...
-
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this product is administered to a nursing woman.
-
Pediatric Use:
Safety and effectiveness in chlidren under the age of 12 years have not ben established.
-
ADVERSE REACTIONSReports of irritation and hypersensitivity to sodium sulfacetamide are uncommon. The following adverse reactions, reported after administration of sterile ophthalmic sodium sulfacetamide, are ...
-
OVERDOSAGE:
The oral LD - 50 of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately. Manifestations: Overdosage may cause nausea and vomiting ...
-
DOSAGE AND ADMINISTRATION:
Seborrheic dermatits including seborrhea sica - Sodium Sulfacetamide 10% Wash: Wash affected areas twice daily (morning and evening), or as directed by your phyiscian. Avoid contact with eyes or ...
-
HOW SUPPLIED:
Sodium Sulfacetamide 10% Wash is supplied in the following size(s): 6 fl. oz. (177 mL) bottle, NDC 16477-410-06, and 12 fl. oz (354.8mL) bottle, NDC 16477-410-12
-
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). See USP Controlled Room Temperature. Protect from freezing. NOTICE: Store upright ...
-
SPL UNCLASSIFIED SECTIONAll prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please NOTE: This is not an Orange Book product and ...
-
PRINCIPAL DISPLAY PANELNDC 16477-410-06 - Rx Only - For External Use Only - Sodium - Sulfacetamide - 10% Wash - LASER Pharmaceuticals, LLC - 6 fl oz (177 mL) INGREDIENTS: Each mL of Sodium Sulfacetamide 10% Wash contains ...
-
INGREDIENTS AND APPEARANCEProduct Information