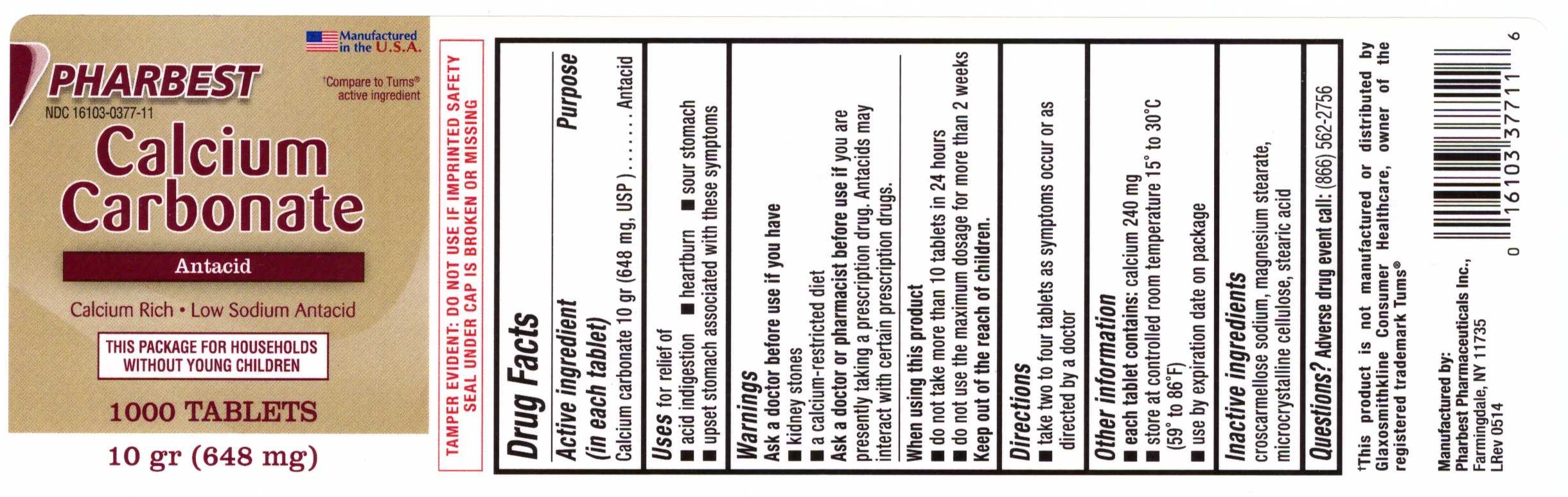
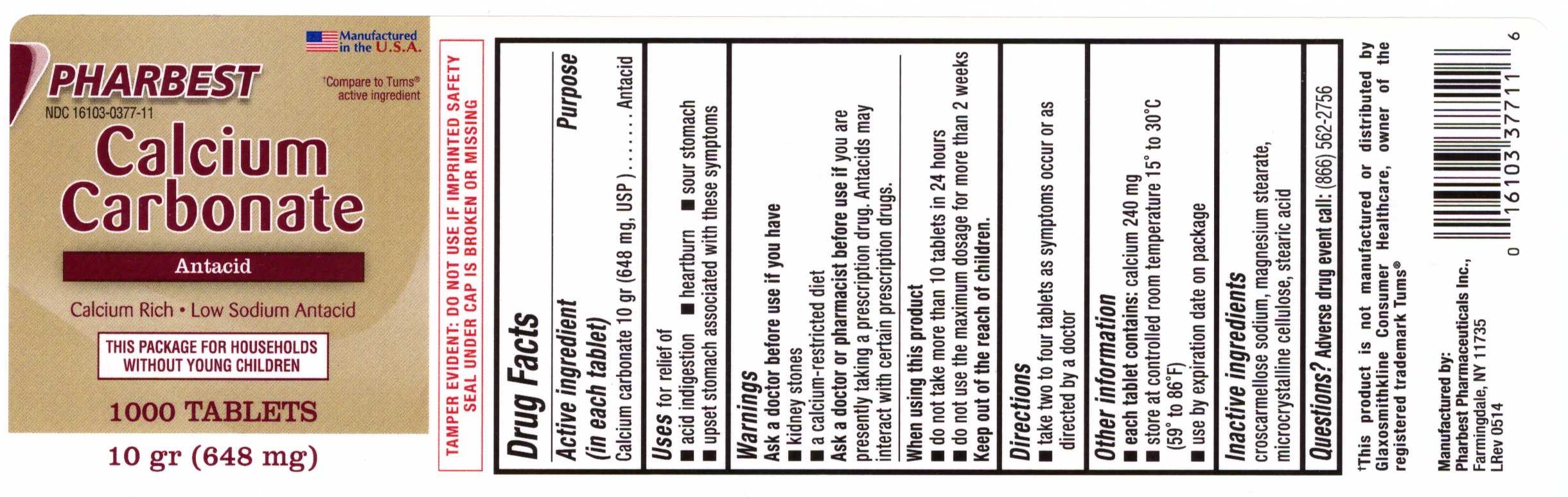
Label: CALCIUM CARBONATE (ANTACID)- calcium carbonate tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 16103-377-08, 16103-377-11 - Packager: Pharbest Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each tablet)
Calcium carbonate 10 gr (648 mg, USP)
-
Purpose
Antacid
-
Uses
for relief of - acid indigestion - heartburn - sour stomach - upset stomach associated with these symptoms
-
Warnings
Ask a doctor before use if you have - kidney stone - a calcium-restricted diet - Ask a doctor or pharmacist before use if - you are presently taking a prescription drug. Antacids may ...
-
Direction
take two to four tablets as symptoms occur or as directed by a doctor
-
Other information
each tablet contains: calcium 240 mg - store at controlled room temperature 150 to 300C (590 to 860F) use by expiration date on package
-
Inactive ingredients
croscarmellose sodium, magnesium stearate, microcrystalline cellulose, stearic acid
-
Questions? Adverse drug event call:
(866) 562-2756
-
PRINCIPAL DISPLAY PANELPHARBEST - NDC 16103-0377-11 - Manufactured in the U.S.A. †Compare to Tums® active ingredient - Calcium - Carbonate - Antacid - Calcium Rich •Low Sodium Antacid - THIS PACKAGE FOR ...
-
INGREDIENTS AND APPEARANCEProduct Information