Label: FELODIPINE tablet, extended release
- NDC Code(s): 13668-132-01, 13668-132-05, 13668-132-10, 13668-132-30, view more
- Packager: Torrent Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
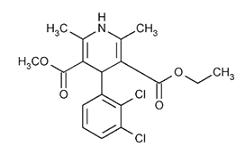
DESCRIPTIONFelodipine is a calcium antagonist (calcium channel blocker). Felodipine is a dihydropyridine derivative that is chemically described as ± ethyl methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2 ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Felodipine is a member of the dihydropyridine class of calcium channel antagonists (calcium channel blockers). It reversibly competes with nitrendipine and/or other ...
-
INDICATIONS AND USAGEFelodipine extended-release tablets are indicated for the treatment of hypertension. Felodipine extended-release tablets may be used alone or concomitantly with other antihypertensive ...
-
CONTRAINDICATIONSFelodipine extended-release tablets are contraindicated in patients who are hypersensitive to this product.
-
PRECAUTIONSGeneral - Hypotension - Felodipine, like other calcium antagonists, may occasionally precipitate significant hypotension and, rarely, syncope. It may lead to reflex tachycardia which in ...
-
Information for PatientsPatients should be instructed to take felodipine extended-release whole and not to crush or chew the tablets. They should be told that mild gingival hyperplasia (gum swelling) has been reported ...
-
Drug InteractionsCYP3A4 Inhibitors - Felodipine is metabolized by CYP3A4. Coadministration of CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, erythromycin, grapefruit juice, cimetidine) with felodipine may ...
-
Carcinogenesis, Mutagenesis, Impairment of FertilityIn a 2 year carcinogenicity study in rats fed felodipine at doses of 7.7, 23.1 or 69.3 mg/kg/day (up to 61 times 1 the maximum recommended human dose on a mg/m 2 basis), a dose related increase ...
-
PregnancyPregnancy Category C - Teratogenic Effects - Studies in pregnant rabbits administered doses of 0.46, 1.2, 2.3 and 4.6 mg/kg/day (from 0.8 to 8 times 1 the maximum recommended human dose on a mg/m 2 ...
-
Nursing MothersIt is not known whether this drug is secreted in human milk and because of the potential for serious adverse reactions from felodipine in the infant, a decision should be made whether to ...
-
Pediatric UseSafety and effectiveness in pediatric patients have not been established.
-
Geriatric UseClinical studies of felodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical ...
-
ADVERSE REACTIONSIn controlled studies in the United States and overseas, approximately 3,000 patients were treated with felodipine as either the extended-release or the immediate-release formulation. The most ...
-
OVERDOSAGEOral doses of 240 mg/kg and 264 mg/kg in male and female mice, respectively, and 2390 mg/kg and 2250 mg/kg in male and female rats, respectively, caused significant lethality. In a suicide ...
-
DOSAGE AND ADMINISTRATIONThe recommended starting dose is 5 mg once a day. Depending on the patient's response, the dosage can be decreased to 2.5 mg or increased to 10 mg once a day. These adjustments should occur ...
-
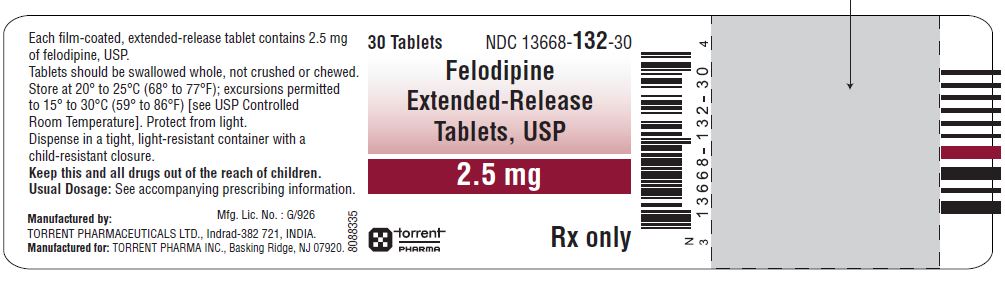
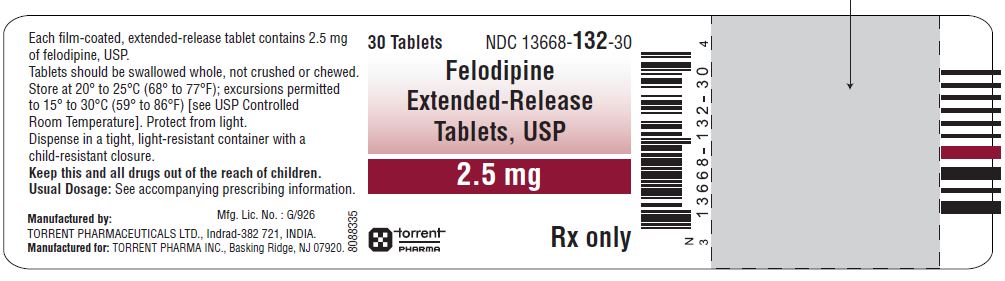
HOW SUPPLIEDFelodipine Extended-Release Tablets, USP are available containing 2.5 mg, 5 mg or 10 mg of felodipine, USP. The 2.5 mg tablet is a green-colored, round-shaped, biconvex film-coated tablets ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELFelodipine Extended-Release Tablets, USP 2.5 mg - Felodipine Extended-Release Tablets, USP 5 mg - Felodipine Extended-Release Tablets, USP 10 mg
-
INGREDIENTS AND APPEARANCEProduct Information