Label: CYCLOSPORINE emulsion
- NDC Code(s): 10702-808-03, 10702-808-06
- Packager: KVK-TECH, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated September 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CYCLOSPORINE OPHTHALMIC EMULSION, 0.05% safely and effectively. See full prescribing information for cyclosporine ophthalmic ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Cyclosporine ophthalmic emulsion, 0.05% is indicated to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with ...
-
2 DOSAGE AND ADMINISTRATION
Invert the unit dose vial a few times to obtain a uniform, white, opaque emulsion before using. Instill one drop of cyclosporine ophthalmic emulsion, 0.05% twice a day in each eye approximately 12 ...
-
3 DOSAGE FORMS AND STRENGTHS
Ophthalmic emulsion containing cyclosporine 0.5 mg/mL
-
4 CONTRAINDICATIONS
Cyclosporine ophthalmic emulsion is contraindicated in patients with known or suspected hypersensitivity to any of the ingredients in the formulation.
-
5 WARNINGS AND PRECAUTIONS
5.1 - Potential for Eye Injury and Contamination - Be careful not to touch the vial tip to your eye or other surfaces to avoid potential for eye injury and ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Potential for Eye Injury and Contamination [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - Clinical administration of cyclosporine ophthalmic emulsion 0.05% is not detected systemically following topical ocular administration [see ...
-
11 DESCRIPTION
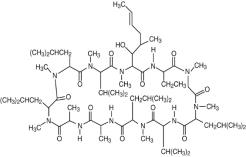
Cyclosporine ophthalmic emulsion, 0.05% contains a topical calcineurin inhibitor immunosuppressant with anti-inflammatory effects. Cyclosporine’s chemical name is ...
-
12 CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Cyclosporine is an immunosuppressive agent when administered systemically. In patients whose tear production is presumed to be suppressed due to ...
-
13 NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Systemic carcinogenicity studies were conducted in male and female mice and rats. In the ...
-
14 CLINICAL STUDIES
Four multicenter, randomized, adequate and well-controlled clinical studies were performed in approximately 1,200 patients with moderate to severe keratoconjunctivitis sicca. Cyclosporine ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Cyclosporine ophthalmic emulsion, 0.05% is packaged in sterile, preservative-free single-use vials. Each vial contains 0.4 mL fill in a 0.9 mL LDPE vial; 30 or 60 vials are packaged in a ...
-
17 PATIENT COUNSELING INFORMATION
Handling the Container - Advise patients to not allow the tip of the vial to touch the eye or any surface, as this may contaminate the emulsion. Advise patients to not touch the vial tip to their ...
-
PRINCIPAL DISPLAY PANEL
NDC 10702-808-03 - Cyclosporine - ophthalmic emulsion 0.05% 30 Single-Use Vials (0.4 mL each) Sterile, Preservative-Free - Rx only
-
PRINCIPAL DISPLAY PANEL
NDC 10702-808-06 - Cyclosporine - ophthalmic emulsion 0.05% 60 Single-Use Vials (0.4 mL each) One Month Supply - Sterile, Preservative-Free - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information