Label: HYDROXYZINE HYDROCHLORIDE tablet, film coated
HYDROXYZINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 10702-010-01, 10702-010-10, 10702-010-50, 10702-011-01, view more
- Packager: KVK-Tech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
DESCRIPTION
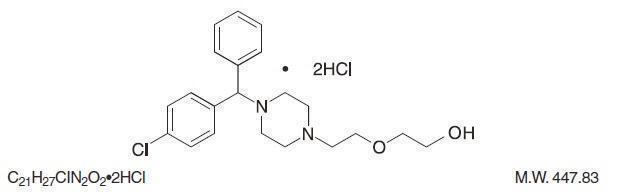
Hydroxyzine hydrochloride has the chemical name of 2-[2-[4-( p-Chloro- α-phenylbenzyl)-1-piperazinyl] ethoxy] ethanol dihydrochloride. Hydroxyzine hydrochloride ...
-
CLINICAL PHARMACOLOGY
Hydroxyzine hydrochloride is unrelated chemically to the phenothiazines, reserpine, meprobamate or the benzodiazepines. Hydroxyzine is not a cortical depressant, but its action may be due to a ...
-
INDICATIONS AND USAGE
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested. Useful in the management of pruritus due to ...
-
CONTRAINDICATIONS
Oral hydroxyzine hydrochloride is contraindicated in patients with known hypersensitivity to hydroxyzine hydrochloride products, and in patients with known hypersensitivity to cetirizine ...
-
WARNINGSNursing Mothers: It is not known whether this drug is excreted in human milk. Since many drugs are so excreted, hydroxyzine should not be given to nursing mothers.
-
PRECAUTIONS
THE POTENTIATING ACTION OF HYDROXYZINE MUST BE CONSIDERED WHEN THE DRUG IS USED IN CONJUNCTION WITH CENTRAL NERVOUS SYSTEM DEPRESSANTS SUCH AS NARCOTICS, NON-NARCOTIC ANALGESICS AND BARBITURATES ...
-
ADVERSE REACTIONS
Side effects reported with the administration of hydroxyzine hydrochloride are usually mild and transitory in nature. Skin and Appendages: Oral hydroxyzine hydrochloride is associated with ...
-
OVERDOSAGE
The most common manifestation of hydroxyzine overdosage is hypersedation. As in the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken. If ...
-
DOSAGE AND ADMINISTRATION
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested: Adults, 50 to 100 mg q.i.d.; children under ...
-
HOW SUPPLIED
Hydroxyzine Hydrochloride Tablets, USP are available as follows: Hydroxyzine hydrochloride tablets, USP 10 mg are supplied as white, round, film coated, biconvex tablets debossed "K10" on one ...
-
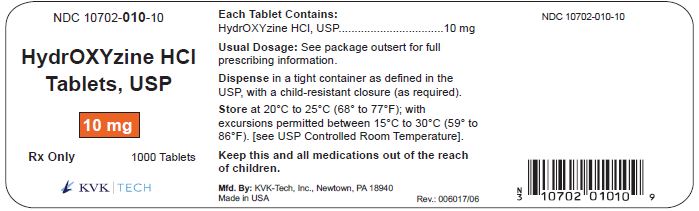
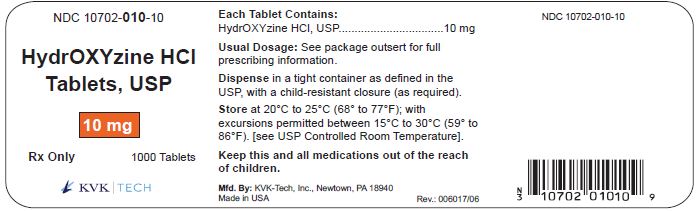
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL – 10 mg Bottle Label
Packaging Size: 100s - NDC 10702-010-01 - HydrOXYzine HCL - Tablets, USP - 10 mg - 100 TABLETS - Rx Only - KVK-TECH, INC. Packaging Size: 500s - NDC 10702-010-50 - HydrOXYzine ...
-
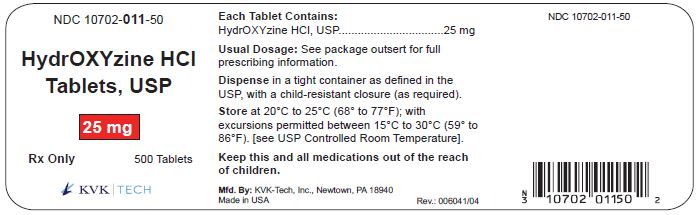
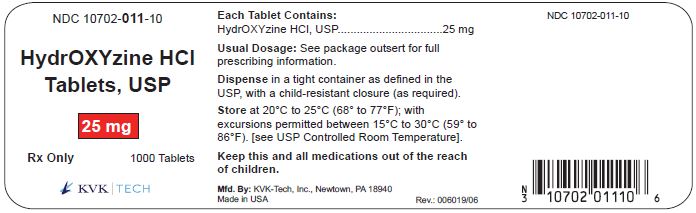
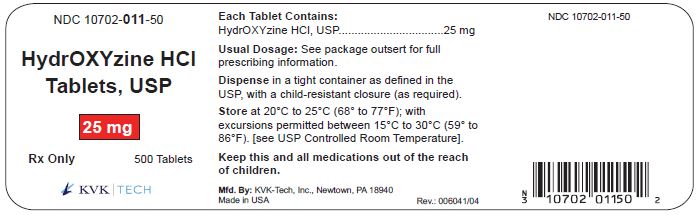
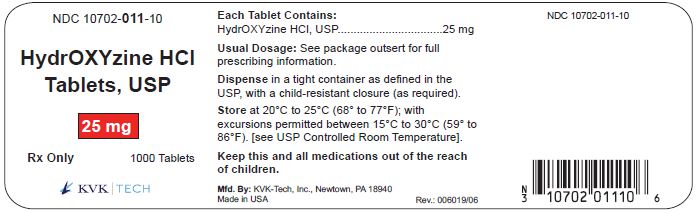
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL – 25 mg Bottle Label
Packaging Size: 100s - NDC 10702-011-01 - HydrOXYzine HCL - Tablets, USP - 25 mg - 100 TABLETS - Rx Only - KVK-TECH, INC. Packaging Size: 500s - NDC 10702-011-50 - HydrOXYzine ...
-
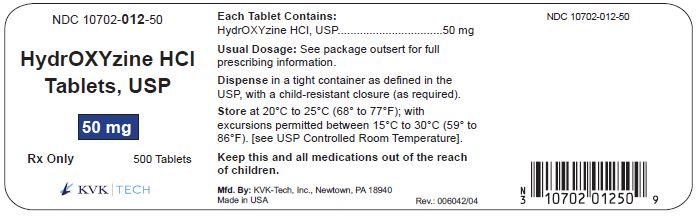
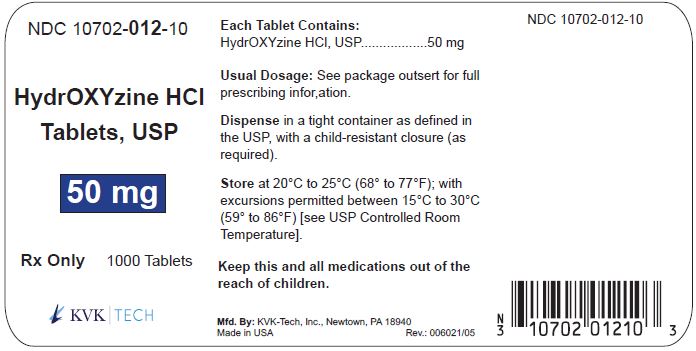
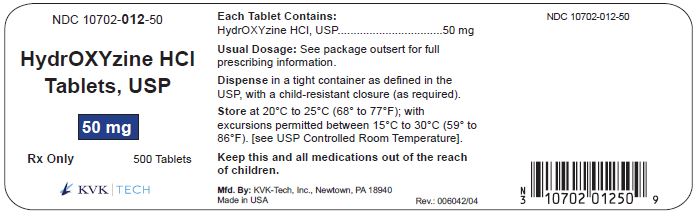
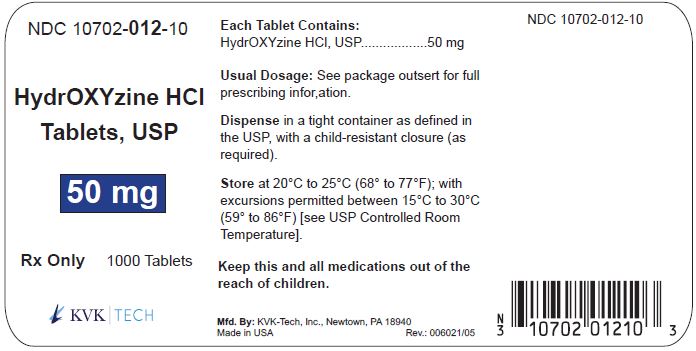
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL – 50 mg Bottle Label
Packaging Size: 100s - NDC 10702-012-01 - HydrOXYzine HCL - Tablets, USP - 50 mg - 100 TABLETS - Rx Only - KVK-TECH, INC. Packaging Size: 500s - NDC 10702-012-50 - HydrOXYzine ...
-
INGREDIENTS AND APPEARANCEProduct Information