Label: SODIUM POLYSTYRENE SULFONATE powder
- NDC Code(s): 10702-036-15, 10702-036-45
- Packager: KVK-Tech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SODIUM POLYSTYRENE SULFONATE safely and effectively. See full prescribing information for SODIUM POLYSTYRENE SULFONATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESodium polystyrene sulfonate is indicated for the treatment of hyperkalemia. Limitation of Use: Sodium polystyrene sulfonate should not be used as an emergency treatment for ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Information - Administer sodium polystyrene sulfonate at least 3 hours before or 3 hours after other oral medications. Patients with gastroparesis may require a 6 hour separation ...
-
3 DOSAGE FORMS AND STRENGTHSSodium polystyrene sulfonate, USP is a cream to light brown, finely ground powder and is available in 454 g jars and 15 g bottles.
-
4 CONTRAINDICATIONSSodium polystyrene sulfonate is contraindicated in patients with the following conditions: • Hypersensitivity to polystyrene sulfonate resins - • Obstructive bowel disease - • Neonates ...
-
5 WARNINGS AND PRECAUTIONS5.1 Intestinal Necrosis - Cases of intestinal necrosis, some fatal, and other serious gastrointestinal adverse events (bleeding, ischemic colitis, perforation) have been reported in association ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed elsewhere in the labeling: • Intestinal Necrosis - [see Warnings and Precautions (5.1)] • Electrolyte Disturbances - [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 General Interactions - No formal drug interaction studies have been conducted in humans. Sodium polystyrene sulfonate has the potential to bind other drugs. In in vitro binding studies ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Sodium polystyrene sulfonate is not absorbed systemically following oral or rectal administration and maternal use is not expected to result in fetal risk ...
-
10 OVERDOSAGEOverdosage may result in electrolyte disturbances including hypokalemia, hypocalcemia, and hypomagnesemia. Appropriate measures should be taken to correct serum electrolytes (potassium, calcium ...
-
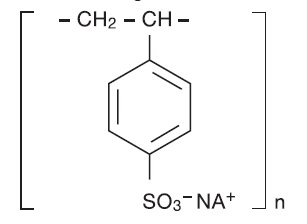
11 DESCRIPTIONSodium polystyrene sulfonate, USP is a benzene, diethenyl-polymer, with ethenylbenzene, sulfonated, sodium salt and has the following structural formula: The drug is a cream to light brown ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sodium polystyrene sulfonate is a non-absorbed, cation exchange polymer that contains a sodium counterion. Sodium polystyrene sulfonate increases fecal potassium ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies have not been performed.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSodium polystyrene sulfonate, USP is available as a cream to light brown, finely ground powder in - Jars of 1 pound (454 g) NDC 10702-036-45 - Bottles of 15 g NDC 10702-036-15 - Store at ...
-
17 PATIENT COUNSELING INFORMATIONDrug Interactions - Advise patients who are taking other oral medication to separate the dosing of sodium polystyrene sulfonate by at least 3 hours (before or after) [see Dosage and ...
-
Principal Display PanelNDC 10702- 036-45 - Sodium Polystyrene Sulfonate, USP - Powder - 454 g (1 lb) Read package outsert. Rx Only - KVK-TECH
-
Principal Display PanelNDC 10702-0036-15 - Sodium Polystyrene Sulfonate, USP - 15 g - Read package outsert. Rx only - KVK-TECH, INC.
-
INGREDIENTS AND APPEARANCEProduct Information