Label: HALOPERIDOL DECANOATE injection
- NDC Code(s): 10147-0921-3, 10147-0922-5
- Packager: Patriot Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated February 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)Increased Mortality in Elderly Patients with Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death ...
WARNING
CloseIncreased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Haloperidol decanoate is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
-
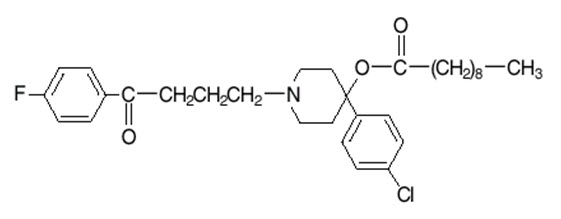
DESCRIPTIONHaloperidol decanoate is the decanoate ester of the butyrophenone, haloperidol. It has a markedly extended duration of effect. It is available in sesame oil in sterile form for intramuscular (IM ...
-
CLINICAL PHARMACOLOGYHaloperidol decanoate 50 and Haloperidol decanoate 100 are the long-acting forms of haloperidol, an antipsychotic. The mechanism of action of haloperidol for the treatment of schizophrenia is ...
-
INDICATIONS AND USAGEHaloperidol decanoate 50 and Haloperidol decanoate 100 are indicated for the treatment of patients with schizophrenia who require prolonged parenteral antipsychotic therapy.
-
CONTRAINDICATIONSSince the pharmacologic and clinical actions of Haloperidol decanoate 50 and Haloperidol decanoate 100 are attributed to haloperidol as the active medication, Contraindications, Warnings, and ...
-
WARNINGSIncreased Mortality in Elderly Patients with Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death ...
-
PRECAUTIONSLeukopenia, Neutropenia, and Agranulocytosis - Class Effect - In clinical trial and/or postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to ...
-
ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: WARNINGS, Increased mortality in Elderly Patients with Dementia-Related Psychosis - WARNINGS ...
-
OVERDOSAGEWhile overdosage is less likely to occur with a parenteral than with an oral medication, information pertaining to haloperidol is presented, modified only to reflect the extended duration of ...
-
DOSAGE AND ADMINISTRATIONHaloperidol decanoate 50 and Haloperidol decanoate 100 should be administered by deep intramuscular injection. A 21 gauge needle is recommended. The maximum volume per injection site should not ...
-
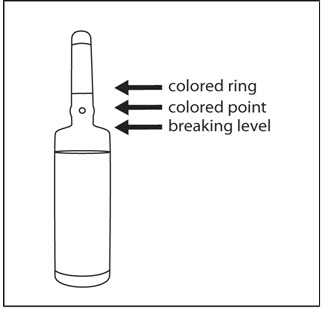
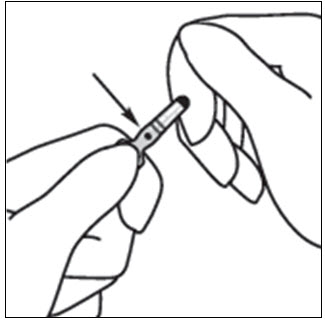
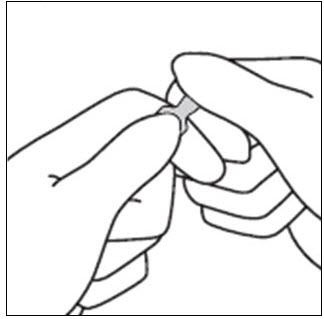
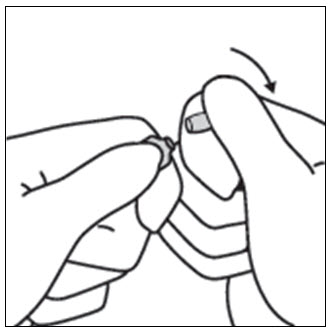
INSTRUCTIONS FOR OPENING AMPULEStep 1 - Medication often rests in the top part of the ampule. Before breaking the ampule, lightly tap the top of the ampule with your finger until all fluid moves to the bottom portion of ...
-
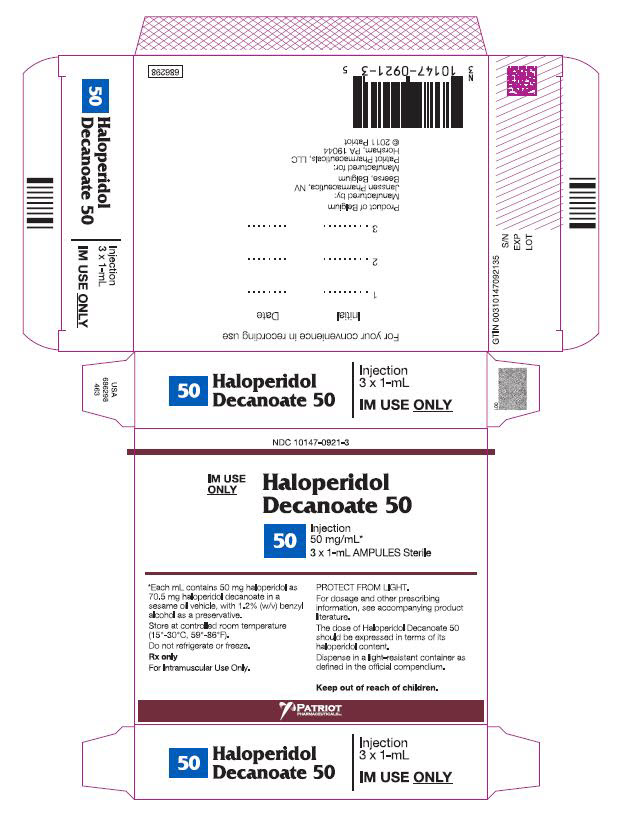
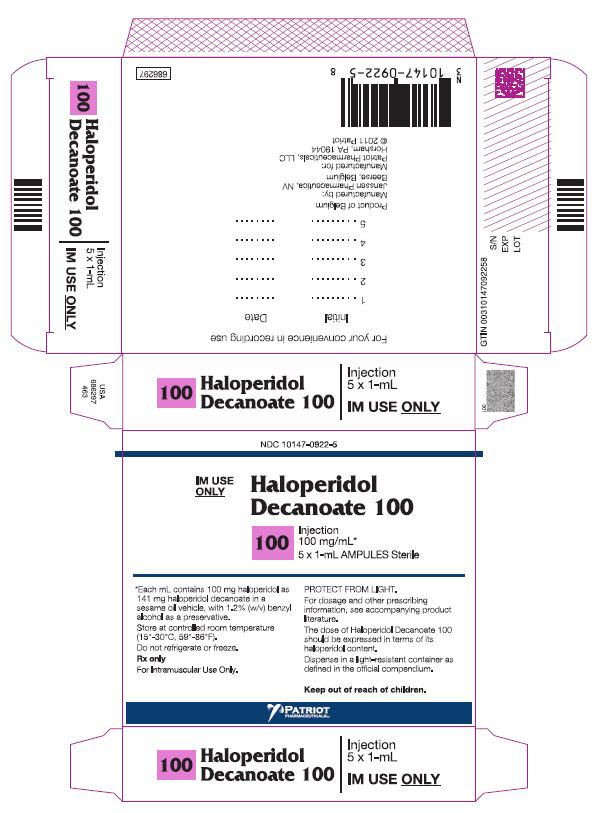
HOW SUPPLIEDHaloperidol decanoate 50 for IM injection, 50 mg haloperidol as 70.52 mg per mL haloperidol decanoate: NDC 10147-0921-3 - 3 × 1 mL ampules. Haloperidol decanoate 100 for IM injection, 100 mg ...
-
SPL UNCLASSIFIED SECTIONProduct of Belgium - Manufactured by: Janssen Pharmaceutica NV - Beerse, Belgium - Or - GlaxoSmithKline Manufacturing S.p.A. Parma, Italy - Manufactured for: Patriot Pharmaceuticals, LLC ...
-
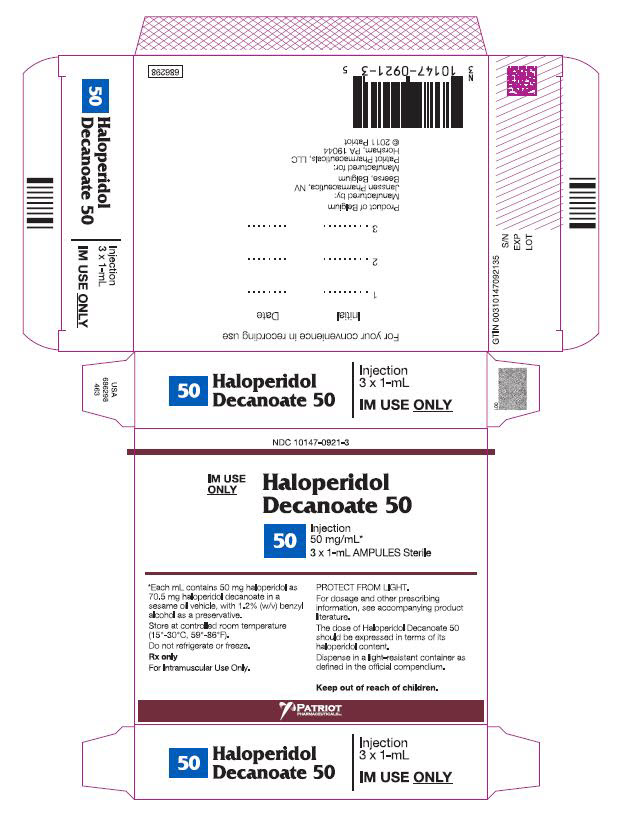
PRINCIPAL DISPLAY PANEL - 50 mg/mL BoxNDC 10147-0921-3 - IM Use - Only - HALOPERIDOL - Decanoate 50 - 50 - INJECTION - 50 mg/mL* 3 x 1-mL AMPULES Sterile - *Each mL contains 50 mg haloperidol - as 70.5 mg ...
-
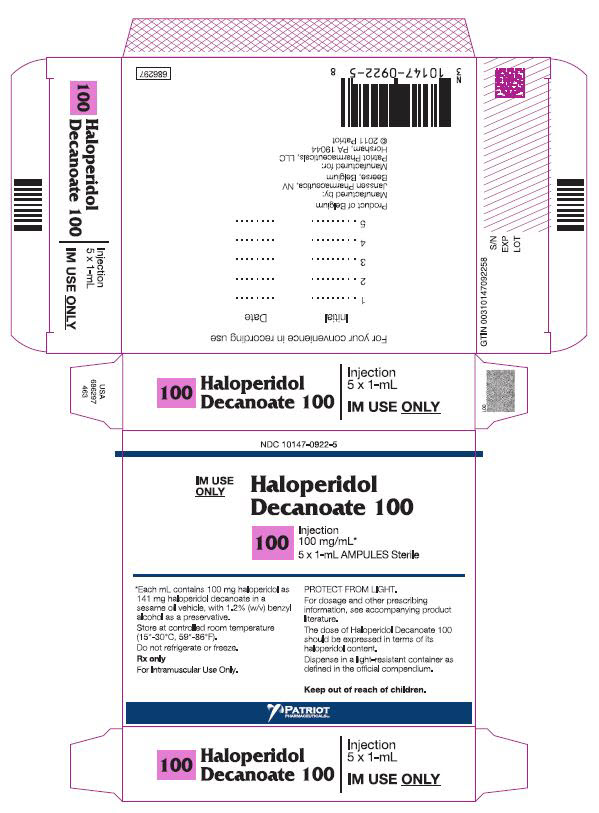
PRINCIPAL DISPLAY PANEL - 100 mg/mL BoxNDC 10147-0922-5 - IM Use - Only - HALOPERIDOL - Decanoate 100 - 100 - INJECTION - 100 mg/mL* 5 x 1-mL AMPULES Sterile - *Each mL contains 100 mg haloperidol - as 141 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information