Label: SORE THROAT LOGENZES SORE THROAT- benzocaine and menthol lozenge
- NDC Code(s): 0904-6255-49
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
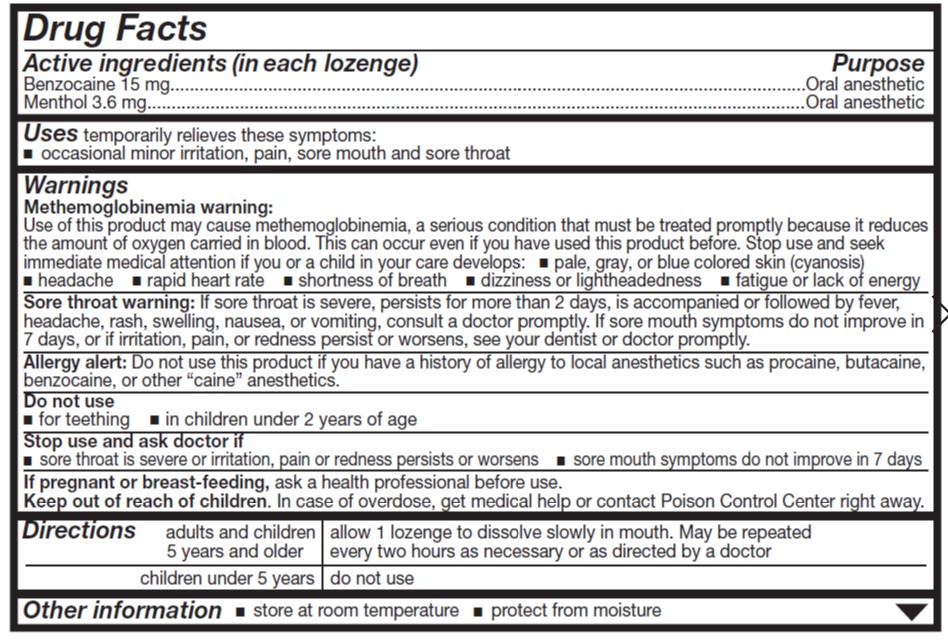
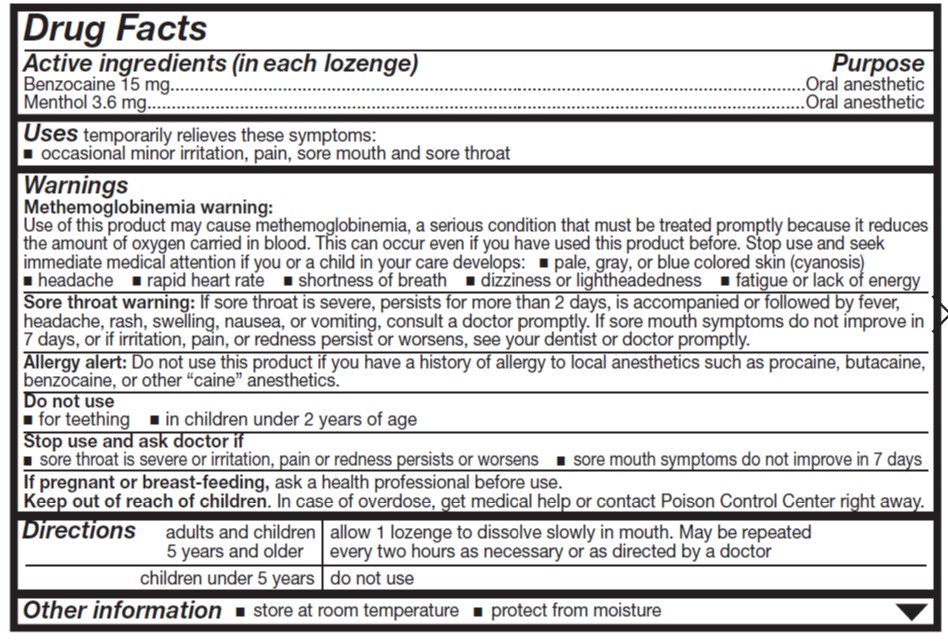
- ACTIVE INGREDIENT
- Uses
-
Warnings
Allergy alert
- Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning
- If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting consult a doctor promptly.
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain or redness persists or worsens
- swelling, rash or fever develops
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
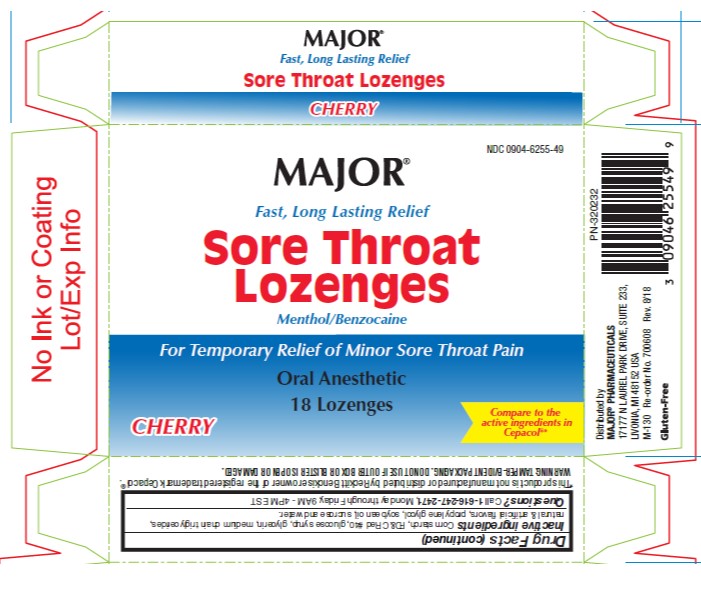
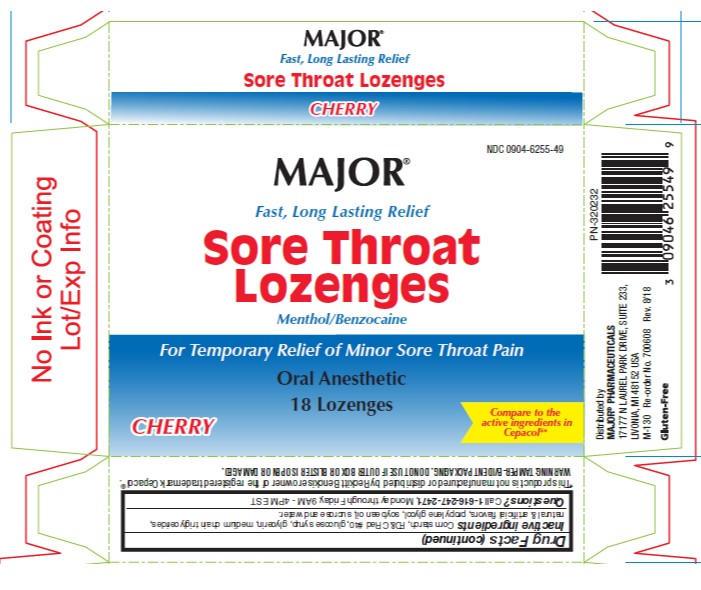
- PRINCIPAL DISPLAY PANEL- 16 Lozenge Carton
-
INGREDIENTS AND APPEARANCE
SORE THROAT LOGENZES SORE THROAT
benzocaine and menthol lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6255 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 15 mg MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 3.6 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) CORN SYRUP (UNII: 9G5L16BK6N) ALLYL SUCROSE (UNII: VI3D59RW0U) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Product Characteristics Color red Score no score Shape ROUND Size 18mm Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6255-49 18 in 1 CARTON 12/28/2012 1 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 12/28/2012 Labeler - Major Pharmaceuticals (191427277) Establishment Name Address ID/FEI Business Operations Bestco, Inc. 002149136 manufacture(0904-6255)