Label: PITAVASTATIN- pitavastatin calcium tablet
- NDC Code(s): 0832-6048-90, 0832-6049-90, 0832-6050-90
- Packager: Upsher-Smith Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PITAVASTATIN TABLETS safely and effectively. See full prescribing information for PITAVASTATIN TABLETS. PITAVASTATIN tablets ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPitavastatin tablets are indicated as an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) in: Adults with primary hyperlipidemia. Adults and pediatric patients aged 8 years ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Information - Take pitavastatin tablets orally once daily with or without food at the same time each day. For patients that require a high-intensity ...
-
3 DOSAGE FORMS AND STRENGTHSPitavastatin tablets are supplied as: 1 mg: Round, white to off-white, film-coated tablet, debossed with "P1" on one side. 2 mg: Round, white to off-white, film-coated tablet, debossed with "P2 ...
-
4 CONTRAINDICATIONSPitavastatin tablets is contraindicated in the following conditions: Concomitant use of cyclosporine - [see - Drug Interactions (7)] . Acute liver failure or decompensated ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myopathy and Rhabdomyolysis - Pitavastatin tablets may cause myopathy (muscle pain, tenderness, or weakness associated with elevated creatine kinase [CK]) and rhabdomyolysis. Acute kidney ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in other sections of the labeling: Myopathy and Rhabdomyolysis - [see - Warnings and Precautions (5.1)] Immune-Mediated ...
-
7 DRUG INTERACTIONSTable 2 includes a list of drugs that increase the risk of myopathy and rhabdomyolysis when administered concomitantly with pitavastatin and instructions for preventing or managing drug ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Discontinue pitavastatin tablets when pregnancy is recognized. Alternatively, consider the ongoing therapeutic needs of the individual patient. Pitavastatin ...
-
10 OVERDOSAGENo specific treatment for pitavastatin tablets overdose is known. Contact Poison Control (1-800-222-1222) for latest recommendations. Hemodialysis is unlikely to be of benefit due to high protein ...
-
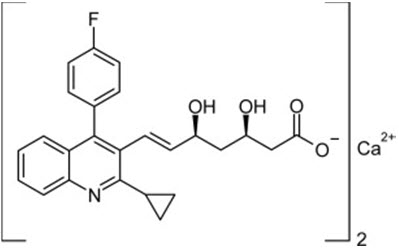
11 DESCRIPTIONPitavastatin tablets for oral use is an HMG-CoA reductase inhibitor. The chemical name for pitavastatin is (+)monocalcium - bis{(3R, 5S, 6 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pitavastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 92-week carcinogenicity study in mice given pitavastatin, at the maximum tolerated dose of 75 mg/kg/day with systemic maximum ...
-
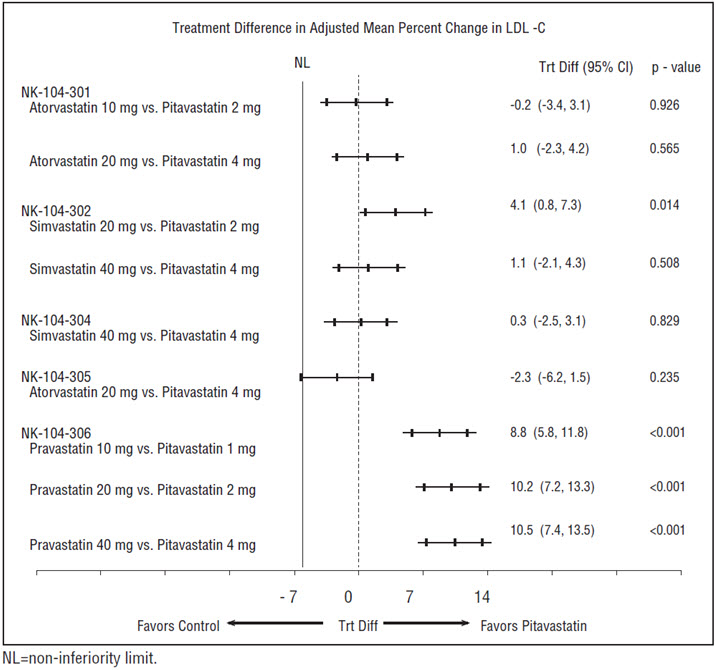
14 CLINICAL STUDIESPrimary Hyperlipidemia in Adults - Study with Atorvastatin (Study 301) Pitavastatin tablets were compared with atorvastatin calcium tablets (referred to as atorvastatin) in a randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPitavastatin tablets, for oral administration are available as: 1 mg: Round, white to off-white, film-coated tablet, debossed with "P1" on one side. They are supplied as: Bottles of 90 with a ...
-
17 PATIENT COUNSELING INFORMATIONMyopathy and Rhabdomyolysis - Advise patients that pitavastatin tablets may cause myopathy and rhabdomyolysis. Inform patients that the risk is increased when taking certain types of medication ...
-
SPL UNCLASSIFIED SECTIONMade in Taiwan - Distributed by - UPSHER-SMITH LABORATORIES, LLC - Maple Grove, MN 55369 - Revised: 4/2024
-
PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle LabelNDC 0832-6048-90 - Pitavastatin - Tablets - 1 mg - 90 Tablets - Rx only - UPSHER-SMITH
-
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle LabelNDC 0832-6049-90 - Pitavastatin - Tablets - 2 mg - 90 Tablets - Rx only - UPSHER-SMITH
-
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Bottle LabelNDC 0832-6050-90 - Pitavastatin - Tablets - 4 mg - 90 Tablets - Rx only - UPSHER-SMITH
-
INGREDIENTS AND APPEARANCEProduct Information