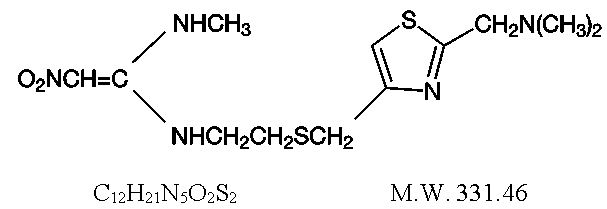
Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H2-receptors, particularly those in the gastric parietal cells.
Antisecretory Activity – 1. Effects on Acid ...
Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H2-receptors, particularly those in the gastric parietal cells.
Antisecretory Activity – 1. Effects on Acid Secretion: Nizatidine significantly inhibited nocturnal gastric acid secretion for up to 12 hours. Nizatidine also significantly inhibited gastric acid secretion stimulated by food, caffeine, betazole, and pentagastrin (Table 1).
2. Effects on Other Gastrointestinal Secretions – Pepsin: Oral administration of 75 to 300 mg of nizatidine did not affect pepsin activity in gastric secretions. Total pepsin output was reduced in proportion to the reduced volume of gastric secretions.
Intrinsic Factor: Oral administration of 75 to 300 mg of nizatidine increased betazole-stimulated secretion of intrinsic factor.
Serum Gastrin: Nizatidine had no effect on basal serum gastrin. No rebound of gastrin secretion was observed when food was ingested 12 hours after administration of nizatidine.
3. Other Pharmacologic Actions
-
Hormones: Nizatidine was not shown to affect the serum concentrations of gonadotropins, prolactin, growth hormone, antidiuretic hormone, cortisol, triiodothyronine, thyroxin, testosterone, 5α-dihydrotestosterone, androstenedione, or estradiol.
- Nizatidine had no demonstrable antiandrogenic action.
4. Pharmacokinetics – The absolute oral bioavailability of nizatidine exceeds 70%. Peak plasma concentrations (700 to 1,800 mcg/L for a 150 mg dose and 1,400 to 3,600 mcg/L for a 300 mg dose) occur from 0.5 to 3 hours following the dose. A concentration of 1,000 mcg/L is equivalent to 3 mcmol/L; a dose of 300 mg is equivalent to 905 mcmoles. Plasma concentrations 12 hours after administration are less than 10 mcg/L. The elimination half-life is 1 to 2 hours, plasma clearance is 40 to 60 L/h, and the volume of distribution is 0.8 to 1.5 L/kg. Because of the short half-life and rapid clearance of nizatidine, accumulation of the drug would not be expected in individuals with normal renal function who take either 300 mg once daily at bedtime or 150 mg twice daily. Nizatidine exhibits dose proportionality over the recommended dose range.
The oral bioavailability of nizatidine is unaffected by concomitant ingestion of propantheline. Antacids consisting of aluminum and magnesium hydroxides with simethicone decrease the absorption of nizatidine by about 10%. With food, the AUC and Cmax increase by approximately 10%.
In humans, less than 7% of an oral dose is metabolized as N2-monodesmethylnizatidine, an H2-receptor antagonist, which is the principal metabolite excreted in the urine. Other likely metabolites are the N2-oxide (less than 5% of the dose) and the S-oxide (less than 6% of the dose).
More than 90% of an oral dose of nizatidine is excreted in the urine within 12 hours. About 60% of an oral dose is excreted as unchanged drug. Renal clearance is about 500 mL/min, which indicates excretion by active tubular secretion. Less than 6% of an administered dose is eliminated in the feces.
Moderate to severe renal impairment significantly prolongs the half-life and decreases the clearance of nizatidine. In individuals who are functionally anephric, the half-life is 3.5 to 11 hours, and the plasma clearance is 7 to 14 L/h. To avoid accumulation of the drug in individuals with clinically significant renal impairment, the amount and/or frequency of doses of nizatidine should be reduced in proportion to the severity of dysfunction (see DOSAGE AND ADMINISTRATION).
Approximately 35% of nizatidine is bound to plasma protein, mainly to α1-acid glycoprotein. Warfarin, diazepam, acetaminophen, propantheline, phenobarbital, and propranolol did not affect plasma protein binding of nizatidine in vitro.
Clinical Trials – 1. Active Duodenal Ulcer: In multicenter, double-blind, placebo-controlled studies in the United States, endoscopically diagnosed duodenal ulcers healed more rapidly following administration of nizatidine, 300 mg h.s. or 150 mg b.i.d., than with placebo (Table 2). Lower doses, such as 100 mg h.s., had slightly lower effectiveness.
2. Maintenance of Healed Duodenal Ulcer: Treatment with a reduced dose of nizatidine has been shown to be effective as maintenance therapy following healing of active duodenal ulcers. In multicenter, double-blind, placebo-controlled studies conducted in the United States, 150 mg of nizatidine taken at bedtime resulted in a significantly lower incidence of duodenal ulcer recurrence in patients treated for up to 1 year (Table 3).
3. Gastroesophageal Reflux Disease (GERD): In 2 multicenter, double-blind, placebo-controlled clinical trials performed in the United States and Canada, nizatidine was more effective than placebo in improving endoscopically diagnosed esophagitis and in healing erosive and ulcerative esophagitis.
In patients with erosive or ulcerative esophagitis, 150 mg b.i.d. of nizatidine given to 88 patients compared with placebo in 98 patients in Study 1 yielded a higher healing rate at 3 weeks (16% vs 7%) and at 6 weeks (32% vs 16%, P<0.05). Of 99 patients on nizatidine and 94 patients on placebo, Study 2 at the same dosage yielded similar results at 6 weeks (21% vs 11%, P <0.05) and at 12 weeks (29% vs 13%, P<0.01).
In addition, relief of associated heartburn was greater in patients treated with nizatidine. Patients treated with nizatidine consumed fewer antacids than did patients treated with placebo.
4. Active Benign Gastric Ulcer: In a multicenter, double-blind, placebo-controlled study conducted in the United States and Canada, endoscopically diagnosed benign gastric ulcers healed significantly more rapidly following administration of nizatidine than of placebo (Table 4).
* P-values are one-sided, obtained by Chi-square test, and not adjusted for multiple comparisons.
In a multicenter, double-blind, comparator-controlled study in Europe, healing rates for patients receiving nizatidine (300 mg h.s. or 150 mg b.i.d.) were equivalent to rates for patients receiving a comparator drug, and statistically superior to historical placebo control rates.
Close