Label: DOBUTAMINE IN DEXTROSE injection, solution
- NDC Code(s): 0409-2346-31, 0409-2346-32, 0409-2347-31, 0409-2347-32, view more
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDOBUTamine - in 5% Dextrose Injection, USP - Flexible Plastic ...
-
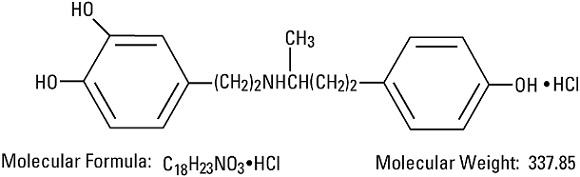
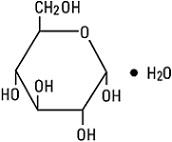
DESCRIPTIONDobutamine in 5% Dextrose Injection, USP is a sterile, nonpyrogenic, prediluted solution of dobutamine hydrochloride and dextrose in water for injection. It is administered by intravenous ...
-
CLINICAL PHARMACOLOGYDobutamine is a direct-acting inotropic agent whose primary activity results from stimulation of the β-receptors of the heart while producing comparatively mild chronotropic, hypertensive ...
-
INDICATIONS AND USAGEDobutamine in 5% Dextrose Injection, USP is indicated when parenteral therapy is necessary for inotropic support in the short-term treatment of patients with cardiac decompensation due to ...
-
CONTRAINDICATIONSDobutamine in 5% Dextrose Injection, USP is contraindicated in patients with idiopathic hypertrophic subaortic stenosis and in patients who have shown previous manifestations of hypersensitivity ...
-
WARNINGSIncrease in Heart Rate or Blood Pressure - Dobutamine hydrochloride may cause a marked increase in heart rate or blood pressure, especially systolic pressure. Approximately 10% of adult patients ...
-
PRECAUTIONSGeneral - During the administration of dobutamine, as with any adrenergic agent, ECG and blood pressure should be continuously monitored. In addition, pulmonary wedge pressure and cardiac output ...
-
ADVERSE REACTIONSIncreased Heart Rate, Blood Pressure, and Ventricular Ectopic Activity: A 10- to 20-mm Hg increase in systolic blood pressure and an increase in heart rate of 5 to 15 beats/minute have been noted ...
-
OVERDOSAGEOverdoses of dobutamine have been reported rarely. The following is provided to serve as a guide if such an overdose is encountered. Signs and Symptoms: Toxicity from dobutamine hydrochloride is ...
-
DOSAGE AND ADMINISTRATIONRecommended Dosage - Dobutamine in 5% Dextrose Injection, USP is administered intravenously through a suitable intravenous catheter or needle. A calibrated electronic infusion device is ...
-
HOW SUPPLIEDDOBUTamine in 5% Dextrose Injection, USP is supplied in 250 LifeCare™ single-dose flexible containers as follows: Unit of Sale - Concentration - NDC 0409-2346-32 - Case of 12 ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - 2,000 mcg/mL250 mL Single-dose container - NDC 0409-2347-31 - DOBUTamine - in 5% Dextrose Injection, USP - 500 mg/250 mL - (2,000 mcg/mL) EACH 100 mL CONTAINS DOBUTAMINE - HYDROCHLORIDE EQUIVALENT TO 200 mg - OF ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Overwrap - 2,000 mcg/mLTO OPEN — TEAR AT NOTCH - 250 mL - NDC 0409-2347-31 - DOBUTamine - in 5% Dextrose Injection, USP - 500 mg/250 mL - (2,000 mcg/mL) Each 100 mL contains Dobutamine Hydrochloride equivalent to 200 mg of ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - 1,000 mcg/mL250 mL Single-dose container - NDC 0409-2346-31 - DOBUTamine - in 5% Dextrose Injection, USP - 250 mg/250 mL - (1,000 mcg/mL) EACH 100 mL CONTAINS DOBUTAMINE - HYDROCHLORIDE EQUIVALENT TO 100 mg - OF ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Overwrap - 1,000 mcg/mLTO OPEN — TEAR AT NOTCH - 250 mL - NDC 0409-2346-31 - DOBUTamine - in 5% Dextrose Injection, USP - 250 mg/250 mL - (1,000 mcg/mL) Each 100 mL contains Dobutamine Hydrochloride equivalent to 100 mg of ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Label - 4,000 mcg/mL250 mL Single-dose container - NDC 0409-3724-11 - DOBUTamine - in 5% Dextrose Injection, USP - 1,000 mg/250 mL - (4,000 mcg/mL) EACH 100 mL CONTAINS DOBUTAMINE - HYDROCHLORIDE EQUIVALENT TO 400 mg - OF ...
-
PRINCIPAL DISPLAY PANEL - 250 mL Bag Overwrap - 4,000 mcg/mLTO OPEN — TEAR AT NOTCH - 250 mL - NDC 0409-3724-11 - DOBUTamine - in 5% Dextrose Injection, USP - 1,000 mg/250 mL - (4,000 mcg/mL) Each 100 mL contains Dobutamine Hydrochloride equivalent to 400 mg of ...
-
INGREDIENTS AND APPEARANCEProduct Information