Label: VALTREX- valacyclovir hydrochloride tablet, film coated
- NDC Code(s): 0173-0565-04, 0173-0565-10, 0173-0933-08, 0173-0933-10
- Packager: GlaxoSmithKline LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VALTREX safely and effectively. See full prescribing information for VALTREX.
VALTREX (valacyclovir) tablets, for oral use
Initial U.S. Approval: 1995INDICATIONS AND USAGE
VALTREX is a deoxynucleoside analogue DNA polymerase inhibitor indicated for:
Adult Patients (1.1)
- •
- Cold Sores (Herpes Labialis)
- •
- Genital Herpes
- •
- Treatment in immunocompetent patients (initial or recurrent episode)
- •
- Suppression in immunocompetent or HIV-1−infected patients
- •
- Reduction of transmission
- •
- Herpes Zoster
Pediatric Patients (1.2)
- •
- Cold Sores (Herpes Labialis)
- •
- Chickenpox
Limitations of Use (1.3)
The efficacy and safety of VALTREX have not been established in immunocompromised patients other than for the suppression of genital herpes in HIV-1−infected patients.
DOSAGE AND ADMINISTRATION
Adult Dosage (2.1)
Cold Sores
2 grams every 12 hours for 1 day
Genital Herpes
Initial episode
1 gram twice daily for 10 days
Recurrent episodes
500 mg twice daily for 3 days
Suppressive therapy
Immunocompetent patients
1 gram once daily
Alternate dose in patients with less than or equal to 9 recurrences/year
500 mg once daily
HIV-1−infected patients
500 mg twice daily
Reduction of transmission
500 mg once daily
Herpes Zoster
1 gram 3 times daily for 7 days
Pediatric Dosage (2.2)
Cold Sores (aged greater than or equal to 12 years)
2 grams every 12 hours for 1 day
Chickenpox (aged 2 to less than 18 years)
20 mg/kg 3 times daily for 5 days; not to exceed 1 gram 3 times daily
Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) can be prepared from the 500 mg VALTREX tablets. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 500 mg (unscored), 1 gram (partially scored) (3)
CONTRAINDICATIONS
Hypersensitivity to valacyclovir (e.g., anaphylaxis), acyclovir, or any component of the formulation. (4)
WARNINGS AND PRECAUTIONS
- •
- Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS): Has occurred in patients with advanced HIV-1 disease and in allogenic bone marrow transplant and renal transplant patients receiving 8 grams per day of VALTREX in clinical trials. Discontinue treatment if clinical symptoms and laboratory findings consistent with TTP/HUS occur. (5.1)
- •
- Acute renal failure: May occur in elderly patients (with or without reduced renal function), patients with underlying renal disease who receive higher-than-recommended doses of VALTREX for their level of renal function, patients who receive concomitant nephrotoxic drugs, or inadequately hydrated patients. Use with caution in elderly patients and reduce dosage in patients with renal impairment. (2.4, 5.2)
- •
- Central nervous system adverse reactions (e.g., agitation, hallucinations, confusion, and encephalopathy): May occur in both adult and pediatric patients (with or without reduced renal function) and in patients with underlying renal disease who receive higher-than-recommended doses of VALTREX for their level of renal function. Elderly patients are more likely to have central nervous system adverse reactions. Use with caution in elderly patients and reduce dosage in patients with renal impairment. (2.4, 5.3)
ADVERSE REACTIONS
- •
- The most common adverse reactions reported in at least one indication by greater than 10% of adult subjects treated with VALTREX and more commonly than in subjects treated with placebo are headache, nausea, and abdominal pain. (6.1)
- •
- The only adverse reaction occurring in greater than 10% of pediatric subjects aged less than 18 years was headache. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adult Patients
1.2 Pediatric Patients
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Adult Dosing Recommendations
2.2 Pediatric Dosing Recommendations
2.3 Extemporaneous Preparation of Oral Suspension
2.4 Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TTP/HUS)
5.2 Acute Renal Failure
5.3 Central Nervous System Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience in Adult Subjects
6.2 Clinical Trials Experience in Pediatric Subjects
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Cold Sores (Herpes Labialis)
14.2 Genital Herpes Infections
14.3 Herpes Zoster
14.4 Chickenpox
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Adult Patients
Cold Sores (Herpes Labialis)
VALTREX is indicated for treatment of cold sores (herpes labialis). The efficacy of VALTREX initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer) has not been established.
Genital Herpes
Initial Episode: VALTREX is indicated for treatment of the initial episode of genital herpes in immunocompetent adults. The efficacy of treatment with VALTREX when initiated more than 72 hours after the onset of signs and symptoms has not been established.
Recurrent Episodes: VALTREX is indicated for treatment of recurrent episodes of genital herpes in immunocompetent adults. The efficacy of treatment with VALTREX when initiated more than 24 hours after the onset of signs and symptoms has not been established.
Suppressive Therapy: VALTREX is indicated for chronic suppressive therapy of recurrent episodes of genital herpes in immunocompetent and in HIV-1−infected adults. The efficacy and safety of VALTREX for the suppression of genital herpes beyond 1 year in immunocompetent patients and beyond 6 months in HIV-1−infected patients have not been established.
Reduction of Transmission: VALTREX is indicated for the reduction of transmission of genital herpes in immunocompetent adults. The efficacy of VALTREX for the reduction of transmission of genital herpes beyond 8 months in discordant couples has not been established. The efficacy of VALTREX for the reduction of transmission of genital herpes in individuals with multiple partners and non‑heterosexual couples has not been established. Safer sex practices should be used with suppressive therapy (see current Centers for Disease Control and Prevention [CDC] Sexually Transmitted Diseases Treatment Guidelines).
Herpes Zoster
VALTREX is indicated for the treatment of herpes zoster (shingles) in immunocompetent adults. The efficacy of VALTREX when initiated more than 72 hours after the onset of rash and the efficacy and safety of VALTREX for treatment of disseminated herpes zoster have not been established.
1.2 Pediatric Patients
Cold Sores (Herpes Labialis)
VALTREX is indicated for the treatment of cold sores (herpes labialis) in pediatric patients aged greater than or equal to 12 years. The efficacy of VALTREX initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer) has not been established.
Chickenpox
VALTREX is indicated for the treatment of chickenpox in immunocompetent pediatric patients aged 2 to less than 18 years. Based on efficacy data from clinical trials with oral acyclovir, treatment with VALTREX should be initiated within 24 hours after the onset of rash [see Clinical Studies (14.4)].
1.3 Limitations of Use
The efficacy and safety of VALTREX have not been established in:
- •
- Immunocompromised patients other than for the suppression of genital herpes in HIV‑1−infected patients with a CD4+ cell count greater than or equal to 100 cells/mm3.
- •
- Patients aged less than 12 years with cold sores (herpes labialis).
- •
- Patients aged less than 2 years or greater than or equal to 18 years with chickenpox.
- •
- Patients aged less than 18 years with genital herpes.
- •
- Patients aged less than 18 years with herpes zoster.
- •
- Neonates and infants as suppressive therapy following neonatal herpes simplex virus (HSV) infection.
-
2 DOSAGE AND ADMINISTRATION
- •
- VALTREX may be given without regard to meals.
- •
- Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) may be prepared extemporaneously from 500-mg VALTREX tablets for use in pediatric patients for whom a solid dosage form is not appropriate [see Dosage and Administration (2.3)].
2.1 Adult Dosing Recommendations
Cold Sores (Herpes Labialis)
The recommended dosage of VALTREX for treatment of cold sores is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning).
Genital Herpes
Initial Episode: The recommended dosage of VALTREX for treatment of initial genital herpes is 1 gram twice daily for 10 days. Therapy was most effective when administered within 48 hours of the onset of signs and symptoms.
Recurrent Episodes: The recommended dosage of VALTREX for treatment of recurrent genital herpes is 500 mg twice daily for 3 days. Initiate treatment at the first sign or symptom of an episode.
Suppressive Therapy: The recommended dosage of VALTREX for chronic suppressive therapy of recurrent genital herpes is 1 gram once daily in patients with normal immune function. In patients with a history of 9 or fewer recurrences per year, an alternative dose is 500 mg once daily.
In HIV‑1−infected patients with a CD4+ cell count greater than or equal to 100 cells/mm3, the recommended dosage of VALTREX for chronic suppressive therapy of recurrent genital herpes is 500 mg twice daily.
Reduction of Transmission: The recommended dosage of VALTREX for reduction of transmission of genital herpes in patients with a history of 9 or fewer recurrences per year is 500 mg once daily for the source partner.
Herpes Zoster
The recommended dosage of VALTREX for treatment of herpes zoster is 1 gram 3 times daily for 7 days. Therapy should be initiated at the earliest sign or symptom of herpes zoster and is most effective when started within 48 hours of the onset of rash.
2.2 Pediatric Dosing Recommendations
Cold Sores (Herpes Labialis)
The recommended dosage of VALTREX for the treatment of cold sores in pediatric patients aged greater than or equal to 12 years is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning).
Chickenpox
The recommended dosage of VALTREX for treatment of chickenpox in immunocompetent pediatric patients aged 2 to less than 18 years is 20 mg/kg administered 3 times daily for 5 days. The total dose should not exceed 1 gram 3 times daily. Therapy should be initiated at the earliest sign or symptom [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
2.3 Extemporaneous Preparation of Oral Suspension
Ingredients and Preparation per USP‑NF
VALTREX tablets 500 mg, cherry flavor, and Suspension Structured Vehicle USP-NF (SSV). Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) should be prepared in lots of 100 mL.
Instructions for Preparing Suspension at Time of Dispensing
- •
- Prepare SSV according to the USP-NF.
- •
- Using a pestle and mortar, grind the required number of VALTREX 500-mg tablets until a fine powder is produced (5 VALTREX tablets for 25-mg/mL suspension; 10 VALTREX tablets for 50-mg/mL suspension).
- •
- Gradually add approximately 5-mL aliquots of SSV to the mortar and triturate the powder until a paste has been produced. Ensure that the powder has been adequately wetted.
- •
- Continue to add approximately 5-mL aliquots of SSV to the mortar, mixing thoroughly between additions, until a concentrated suspension is produced, to a minimum total quantity of 20 mL SSV and a maximum total quantity of 40 mL SSV for both the 25-mg/mL and 50-mg/mL suspensions.
- •
- Transfer the mixture to a suitable 100-mL measuring flask.
- •
- Transfer the cherry flavor* to the mortar and dissolve in approximately 5 mL of SSV. Once dissolved, add to the measuring flask.
- •
- Rinse the mortar at least 3 times with approximately 5-mL aliquots of SSV, transferring the rinsing to the measuring flask between additions.
- •
- Make the suspension to volume (100 mL) with SSV and shake thoroughly to mix.
- •
- Transfer the suspension to an amber glass medicine bottle with a child-resistant closure.
- •
- The prepared suspension should be labeled with the following information “Shake well before using. Store suspension between 2°C to 8°C (36°F to 46°F) in a refrigerator. Discard after 28 days.”
*The amount of cherry flavor added is as instructed by the suppliers of the cherry flavor.
2.4 Patients with Renal Impairment
Dosage recommendations for adult patients with reduced renal function are provided in Table 1 [see Use in Specific Populations (8.5, 8.6), Clinical Pharmacology (12.3)]. Data are not available for the use of VALTREX in pediatric patients with a creatinine clearance less than 50 mL/min/1.73 m2.
Table 1. VALTREX Dosage Recommendations for Adults with Renal Impairment Indications
Normal Dosage
Regimen
(Creatinine Clearance ≥50 mL/min)
Creatinine Clearance (mL/min)
30-49
10-29
<10
Cold sores (Herpes Labialis)
Do not exceed 1 day of treatment.
Two 2-gram doses taken 12 hours apart
Two 1-gram doses taken 12 hours apart
Two 500-mg doses taken 12 hours apart
500-mg single dose
Genital herpes:
Initial episode
1 gram every 12 hours
no reduction
1 gram every 24 hours
500 mg every 24 hours
Genital herpes:
Recurrent episode
500 mg every 12 hours
no reduction
500 mg every 24 hours
500 mg every 24 hours
Genital herpes:
Suppressive therapy
Immunocompetent patients
1 gram every 24 hours
no reduction
500 mg every 24 hours
500 mg every 24 hours
Alternate dose for immunocompetent patients with less than or equal to 9 recurrences/year
500 mg every 24 hours
no reduction
500 mg every 48 hours
500 mg every 48 hours
HIV‑1−infected patients
500 mg every 12 hours
no reduction
500 mg every 24 hours
500 mg every 24 hours
Herpes zoster
1 gram every 8 hours
1 gram every 12 hours
1 gram every 24 hours
500 mg every 24 hours
Hemodialysis
Patients requiring hemodialysis should receive the recommended dose of VALTREX after hemodialysis. During hemodialysis, the half-life of acyclovir after administration of VALTREX is approximately 4 hours. About one-third of acyclovir in the body is removed by dialysis during a 4-hour hemodialysis session.
Peritoneal Dialysis
There is no information specific to administration of VALTREX in patients receiving peritoneal dialysis. The effect of chronic ambulatory peritoneal dialysis (CAPD) and continuous arteriovenous hemofiltration/dialysis (CAVHD) on acyclovir pharmacokinetics has been studied. The removal of acyclovir after CAPD and CAVHD is less pronounced than with hemodialysis, and the pharmacokinetic parameters closely resemble those observed in patients with end-stage renal disease (ESRD) not receiving hemodialysis. Therefore, supplemental doses of VALTREX should not be required following CAPD or CAVHD.
-
3 DOSAGE FORMS AND STRENGTHS
Tablets:
- •
- 500-mg: Each blue, film‑coated, capsule‑shaped tablet printed with “VALTREX 500 mg” contains 556.2 mg of valacyclovir hydrochloride equivalent to 500 mg of the free base.
- •
- 1-gram: Each blue, film‑coated, capsule‑shaped tablet, with a partial scorebar on both sides, printed with “VALTREX 1 gram” contains 1.112 grams of valacyclovir hydrochloride equivalent to 1 gram of the free base.
-
4 CONTRAINDICATIONS
VALTREX is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction (e.g., anaphylaxis) to valacyclovir, acyclovir, or any component of the formulation [see Adverse Reactions (6.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TTP/HUS)
TTP/HUS, in some cases resulting in death, has occurred in patients with advanced HIV-1 disease and also in allogeneic bone marrow transplant and renal transplant recipients participating in clinical trials of VALTREX at doses of 8 grams per day. Treatment with VALTREX should be stopped immediately if clinical signs, symptoms, and laboratory abnormalities consistent with TTP/HUS occur.
5.2 Acute Renal Failure
Cases of acute renal failure have been reported in:
- •
- Elderly patients with or without reduced renal function. Caution should be exercised when administering VALTREX to geriatric patients, and dosage reduction is recommended for those with impaired renal function [see Dosage and Administration (2.4), Use in Specific Populations (8.5)].
- •
- Patients with underlying renal disease who received higher-than-recommended doses of VALTREX for their level of renal function. Dosage reduction is recommended when administering VALTREX to patients with renal impairment [see Dosage and Administration (2.4), Use in Specific Populations (8.6)].
- •
- Patients receiving other nephrotoxic drugs. Caution should be exercised when administering VALTREX to patients receiving potentially nephrotoxic drugs.
- •
- Patients without adequate hydration. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. Adequate hydration should be maintained for all patients.
In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored [see Dosage and Administration (2.4), Adverse Reactions (6.3)].
5.3 Central Nervous System Effects
Central nervous system adverse reactions, including agitation, hallucinations, confusion, delirium, seizures, and encephalopathy, have been reported in both adult and pediatric patients with or without reduced renal function and in patients with underlying renal disease who received higher-than-recommended doses of VALTREX for their level of renal function. Elderly patients are more likely to have central nervous system adverse reactions. VALTREX should be discontinued if central nervous system adverse reactions occur [see Adverse Reactions (6.3), Use in Specific Populations (8.5, 8.6)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome [see Warnings and Precautions (5.1)].
- •
- Acute Renal Failure [see Warnings and Precautions (5.2)].
- •
- Central Nervous System Effects [see Warnings and Precautions (5.3)].
The most common adverse reactions reported in at least 1 indication by greater than 10% of adult subjects treated with VALTREX and observed more frequently with VALTREX compared with placebo are headache, nausea, and abdominal pain. The only adverse reaction reported in greater than 10% of pediatric subjects aged less than 18 years was headache.
6.1 Clinical Trials Experience in Adult Subjects
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cold Sores (Herpes Labialis)
In clinical trials for the treatment of cold sores, the adverse reactions reported by subjects receiving VALTREX 2 grams twice daily (n = 609) or placebo (n = 609) for 1 day, respectively, included headache (14%, 10%) and dizziness (2%, 1%). The frequencies of abnormal ALT (greater than 2 x ULN) were 1.8% for subjects receiving VALTREX compared with 0.8% for placebo. Other laboratory abnormalities (hemoglobin, white blood cells, alkaline phosphatase, and serum creatinine) occurred with similar frequencies in the 2 groups.
Genital Herpes
Initial Episode: In a clinical trial for the treatment of initial episodes of genital herpes, the adverse reactions reported by greater than or equal to 5% of subjects receiving VALTREX 1 gram twice daily for 10 days (n = 318) or oral acyclovir 200 mg 5 times daily for 10 days (n = 318), respectively, included headache (13%, 10%) and nausea (6%, 6%). For the incidence of laboratory abnormalities see Table 2.
Recurrent Episodes: In 3 clinical trials for the episodic treatment of recurrent genital herpes, the adverse reactions reported by greater than or equal to 5% of subjects receiving VALTREX 500 mg twice daily for 3 days (n = 402), VALTREX 500 mg twice daily for 5 days (n = 1,136), or placebo (n = 259), respectively, included headache (16%, 11%, 14%) and nausea (5%, 4%, 5%). For the incidence of laboratory abnormalities see Table 2.
Suppressive Therapy: Suppression of Recurrent Genital Herpes in Immunocompetent Adults: In a clinical trial for the suppression of recurrent genital herpes infections, the adverse reactions reported by subjects receiving VALTREX 1 gram once daily (n = 269), VALTREX 500 mg once daily (n = 266), or placebo (n = 134), respectively, included headache (35%, 38%, 34%), nausea (11%, 11%, 8%), abdominal pain (11%, 9%, 6%), dysmenorrhea (8%, 5%, 4%), depression (7%, 5%, 5%), arthralgia (6%, 5%, 4%), vomiting (3%, 3%, 2%), and dizziness (4%, 2%, 1%). For the incidence of laboratory abnormalities see Table 2.
Suppression of Recurrent Genital Herpes in HIV-1–Infected Subjects: In HIV-1–infected subjects, frequently reported adverse reactions for VALTREX (500 mg twice daily; n = 194, median days on therapy = 172) and placebo (n = 99, median days on therapy = 59), respectively, included headache (13%, 8%), fatigue (8%, 5%), and rash (8%, 1%). Post-randomization laboratory abnormalities that were reported more frequently in valacyclovir subjects versus placebo included elevated alkaline phosphatase (4%, 2%), elevated ALT (14%, 10%), elevated AST (16%, 11%), decreased neutrophil counts (18%, 10%), and decreased platelet counts (3%, 0%), respectively.
Reduction of Transmission: In a clinical trial for the reduction of transmission of genital herpes, the adverse reactions reported by subjects receiving VALTREX 500 mg once daily (n = 743) or placebo once daily (n = 741), respectively, included headache (29%, 26%), nasopharyngitis (16%, 15%), and upper respiratory tract infection (9%, 10%).
Herpes Zoster
In 2 clinical trials for the treatment of herpes zoster, the adverse reactions reported by subjects receiving VALTREX 1 gram 3 times daily for 7 to 14 days (n = 967) or placebo (n = 195), respectively, included nausea (15%, 8%), headache (14%, 12%), vomiting (6%, 3%), dizziness (3%, 2%), and abdominal pain (3%, 2%). For the incidence of laboratory abnormalities see Table 2.
Table 2. Incidence (%) of Laboratory Abnormalities in Herpes Zoster and Genital Herpes Trial Populations a Data were not collected prospectively.
LLN = Lower limit of normal.
ULN = Upper limit of normal.Laboratory Abnormality
Herpes Zoster
Genital Herpes Treatment
Genital Herpes Suppression
VALTREX
1 gram
3 Times Daily
(n = 967)
Place-bo
(n = 195)
VALTREX 1 gram Twice Daily
(n = 1,194)
VALTREX 500 mg Twice Daily
(n = 1,159)
Place-bo
(n = 439)
VALTREX
1 gram Once Daily
(n = 269)
VALTREX 500 mg Once Daily
(n = 266)
Place-bo
(n = 134)
Hemoglobin
(<0.8 x LLN)
0.8%
0%
0.3%
0.2%
0%
0%
0.8%
0.8%
White blood cells
(<0.75 x LLN)
1.3%
0.6%
0.7%
0.6%
0.2%
0.7%
0.8%
1.5%
Platelet count (<100,000/mm3)
1.0%
1.2%
0.3%
0.1%
0.7%
0.4%
1.1%
1.5%
AST (SGOT)
(>2 x ULN)
1.0%
0%
1.0%
a
0.5%
4.1%
3.8%
3.0%
Serum creatinine
(>1.5 x ULN)
0.2%
0%
0.7%
0%
0%
0%
0%
0%
6.2 Clinical Trials Experience in Pediatric Subjects
The safety profile of VALTREX has been studied in 177 pediatric subjects aged 1 month to less than 18 years. Sixty-five of these pediatric subjects, aged 12 to less than 18 years, received oral tablets for 1 to 2 days for treatment of cold sores. The remaining 112 pediatric subjects, aged 1 month to less than 12 years, participated in 3 pharmacokinetic and safety trials and received valacyclovir oral suspension. Fifty-one of these 112 pediatric subjects received oral suspension for 3 to 6 days. The frequency, intensity, and nature of clinical adverse reactions and laboratory abnormalities were similar to those seen in adults.
Pediatric Subjects Aged 12 to Less than 18 Years (Cold Sores)
In clinical trials for the treatment of cold sores, the adverse reactions reported by adolescent subjects receiving VALTREX 2 grams twice daily for 1 day, or VALTREX 2 grams twice daily for 1 day followed by 1 gram twice daily for 1 day (n = 65, across both dosing groups), or placebo (n = 30), respectively, included headache (17%, 3%) and nausea (8%, 0%).
Pediatric Subjects Aged 1 Month to Less than 12 Years
Adverse events reported in more than 1 subject across the 3 pharmacokinetic and safety trials in children aged 1 month to less than 12 years were diarrhea (5%), pyrexia (4%), dehydration (2%), herpes simplex (2%), and rhinorrhea (2%). No clinically meaningful changes in laboratory values were observed.
6.3 Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during postmarketing use of VALTREX. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to VALTREX.
General
Facial edema, hypertension, tachycardia.
Allergic
Acute hypersensitivity reactions including anaphylaxis, angioedema, dyspnea, pruritus, rash, and urticaria [see Contraindications (4)].
Central Nervous System (CNS) Symptoms
Aggressive behavior; agitation; ataxia; coma; confusion; decreased consciousness; dysarthria; encephalopathy; mania; and psychosis, including auditory and visual hallucinations, seizures, tremors [see Warnings and Precautions (5.3), Use in Specific Populations (8.5, 8.6)].
Eye
Visual abnormalities.
Gastrointestinal
Diarrhea.
Hepatobiliary Tract and Pancreas
Liver enzyme abnormalities, hepatitis.
Renal
Renal failure, renal pain (may be associated with renal failure) [see Warnings and Precautions (5.2), Use in Specific Populations (8.5, 8.6)].
Hematologic
Thrombocytopenia, aplastic anemia, leukocytoclastic vasculitis, TTP/HUS [see Warnings and Precautions (5.1)].
Skin
Erythema multiforme, rashes including photosensitivity, alopecia.
-
7 DRUG INTERACTIONS
No clinically significant drug-drug or drug-food interactions with VALTREX are known [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Clinical data over several decades with valacyclovir and its metabolite, acyclovir, in pregnant women, have not identified a drug associated risk of major birth defects. There are insufficient data on the use of valacyclovir regarding miscarriage or adverse maternal or fetal outcomes (see Data). There are risks to the fetus associated with untreated herpes simplex during pregnancy (see Clinical Considerations).
In animal reproduction studies, no evidence of adverse developmental outcomes was observed with valacyclovir when administered to pregnant rats and rabbits at system exposures (AUC) 4 (rats) and 7 (rabbits) times the human exposure at the maximum recommended human dose (MRHD) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: The risk of neonatal HSV infection varies from 30% to 50% for genital HSV acquired in late pregnancy (third trimester), whereas with HSV acquisition in early pregnancy, the risk of neonatal infection is about 1%. A primary herpes occurrence during the first trimester of pregnancy has been associated with neonatal chorioretinitis, microcephaly, and, in rare cases, skin lesions. In very rare cases, transplacental transmission can occur resulting in congenital infection, including microcephaly, hepatosplenomegaly, intrauterine growth restriction, and stillbirth. Co-infection with HSV increases the risk of perinatal HIV transmission in women who had a clinical diagnosis of genital herpes during pregnancy.
Data
Human Data: Clinical data over several decades with valacyclovir and its metabolite, acyclovir, in pregnant women, based on published literature, have not identified a drug-associated risk of major birth defects. There are insufficient data on the use of valacyclovir regarding miscarriage or adverse maternal or fetal outcomes.
The Acyclovir and the Valacyclovir Pregnancy Registries, both population-based international prospective studies, collected pregnancy data through April 1999. The Acyclovir Registry documented outcomes of 1,246 infants and fetuses exposed to acyclovir during pregnancy (756 with earliest exposure during the first trimester, 197 during the second trimester, 291 during the third trimester, and 2 unknown). The occurrence of major birth defects during first-trimester exposure to acyclovir was 3.2% (95% CI: 2.0% to 5.0%) and during any trimester of exposure was 2.6% (95% CI: 1.8% to 3.8%). The Valacyclovir Pregnancy Registry documented outcomes of 111 infants and fetuses exposed to valacyclovir during pregnancy (28 with earliest exposure in the first trimester, 31 during the second trimester, and 52 during the third trimester).The occurrence of major birth defects during first-trimester exposure to valacyclovir was 4.5% (95% CI: 0.24% to 24.9%) and during any trimester of exposure was 3.9% (95% CI: 1.3% to 10.7%).
Available studies have methodological limitations including insufficient sample size to support conclusions about overall malformation risk or for making comparisons of the frequencies of specific birth defects.
Animal Data: Valacyclovir was administered orally to pregnant rats and rabbits (up to 400 mg/kg/day) during organogenesis (Gestation Days 6 through 15, and 6 through 18, respectively). No adverse embryo-fetal effects were observed in rats and rabbits at acyclovir exposures (AUC) of up to approximately 4 (rats) and 7 (rabbits) times the exposure in humans at the MRHD. Early embryo death, fetal growth retardation (weight and length), and variations in fetal skeletal development (primarily extra ribs and delayed ossification of sternebrae) were observed in rats and associated with maternal toxicity (200 mg/kg/day; approximately 6 times higher than human exposure at the MRHD).
In a pre/postnatal development study, valacyclovir was administered orally to pregnant rats (up to 200 mg/kg/day from Gestation Day 15 to Post-Partum Day 20) from late gestation through lactation. No significant adverse effects were observed in offspring exposed daily from before birth through lactation at maternal exposures (AUC) of approximately 6 times higher than human exposures at the MRHD.
8.2 Lactation
Risk Summary
Although there is no information on the presence of valacyclovir in human milk, its metabolite, acyclovir, is present in human milk following oral administration of valacyclovir. Based on published data, a 500-mg maternal dose of VALTREX twice daily would provide a breastfed child with an oral acyclovir dosage of approximately 0.6 mg/kg/day (see Data). There is no data on the effects of valacyclovir or acyclovir on the breastfed child or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VALTREX and any potential adverse effects on the breastfed child from VALTREX or from the underlying maternal condition.
Data
Following oral administration of a 500-mg dose of VALTREX to 5 lactating women, peak acyclovir concentrations (Cmax) in breast milk ranged from 0.5 to 2.3 times (median 1.4) the corresponding maternal acyclovir serum concentrations. The acyclovir breast milk AUC ranged from 1.4 to 2.6 times (median 2.2) maternal serum AUC. A 500-mg maternal dose of VALTREX twice daily would provide a breastfed child with an oral acyclovir dosage of approximately 0.6 mg/kg/day. Unchanged valacyclovir was not detected in maternal serum, breast milk or infant urine.
8.4 Pediatric Use
VALTREX is indicated for treatment of cold sores in pediatric patients aged greater than or equal to 12 years and for treatment of chickenpox in pediatric patients aged 2 to less than 18 years [see Indications and Usage (1.2), Dosage and Administration (2.2)].
The use of VALTREX for treatment of cold sores is based on 2 double‑blind, placebo‑controlled clinical trials in healthy adults and adolescents (aged greater than or equal to 12 years) with a history of recurrent cold sores [see Clinical Studies (14.1)].
The use of VALTREX for treatment of chickenpox in pediatric patients aged 2 to less than 18 years is based on single‑dose pharmacokinetic and multiple‑dose safety data from an open‑label trial with valacyclovir and supported by efficacy and safety data from 3 randomized, double‑blind, placebo‑controlled trials evaluating oral acyclovir in pediatric subjects with chickenpox [see Dosage and Administration (2.2), Adverse Reactions (6.2), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
The efficacy and safety of valacyclovir have not been established in pediatric patients:
- •
- aged less than 12 years with cold sores
- •
- aged less than 18 years with genital herpes
- •
- aged less than 18 years with herpes zoster
- •
- aged less than 2 years with chickenpox
- •
- for suppressive therapy following neonatal HSV infection.
The pharmacokinetic profile and safety of valacyclovir oral suspension in children aged less than 12 years were studied in 3 open‑label trials. No efficacy evaluations were conducted in any of the 3 trials.
Trial 1 was a single‑dose pharmacokinetic, multiple‑dose safety trial in 27 pediatric subjects aged 1 to less than 12 years with clinically suspected varicella‑zoster virus (VZV) infection [see Dosage and Administration (2.2), Adverse Reactions (6.2), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
Trial 2 was a single‑dose pharmacokinetic and safety trial in pediatric subjects aged 1 month to less than 6 years who had an active herpes virus infection or who were at risk for herpes virus infection. Fifty‑seven subjects were enrolled and received a single dose of 25 mg/kg valacyclovir oral suspension. In infants and children aged 3 months to less than 6 years, this dose provided comparable systemic acyclovir exposures to that from a 1‑gram dose of valacyclovir in adults (historical data). In infants aged 1 month to less than 3 months, mean acyclovir exposures resulting from a 25‑mg/kg dose were higher (Cmax: ↑30%, AUC: ↑60%) than acyclovir exposures following a 1‑gram dose of valacyclovir in adults. Acyclovir is not approved for suppressive therapy in infants and children following neonatal HSV infections; therefore, valacyclovir is not recommended for this indication because efficacy cannot be extrapolated from acyclovir.
Trial 3 was a single‑dose pharmacokinetic, multiple‑dose safety trial in 28 pediatric subjects aged 1 to less than 12 years with clinically suspected HSV infection. None of the subjects enrolled in this trial had genital herpes. Each subject was dosed with valacyclovir oral suspension 10 mg/kg twice daily for 3 to 5 days. Acyclovir systemic exposures in pediatric subjects following valacyclovir oral suspension were compared with historical acyclovir systemic exposures in immunocompetent adults receiving the solid oral dosage form of valacyclovir or acyclovir for the treatment of recurrent genital herpes. The mean projected daily acyclovir systemic exposures in pediatric subjects across all age‑groups (1 to less than 12 years) were lower (Cmax: ↓20%, AUC: ↓33%) compared with the acyclovir systemic exposures in adults receiving valacyclovir 500 mg twice daily but were higher (daily AUC: ↑16%) than systemic exposures in adults receiving acyclovir 200 mg 5 times daily. Insufficient data are available to support valacyclovir for the treatment of recurrent genital herpes in this age‑group because clinical information on recurrent genital herpes in young children is limited; therefore, extrapolating efficacy data from adults to this population is not possible. Moreover, valacyclovir has not been studied in children aged 1 to less than 12 years with recurrent genital herpes.
8.5 Geriatric Use
Of the total number of subjects in clinical trials of VALTREX, 906 were 65 and over, and 352 were 75 and over. In a clinical trial of herpes zoster, the duration of pain after healing (post-herpetic neuralgia) was longer in subjects 65 and older compared with younger adults. Elderly patients are more likely to have reduced renal function and require dose reduction. Elderly patients are also more likely to have renal or CNS adverse events [see Dosage and Administration (2.4), Warnings and Precautions (5.2, 5.3), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Caution should be exercised to prevent inadvertent overdose [see Use in Specific Populations (8.5, 8.6)]. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored [see Dosage and Administration (2.4)].
-
11 DESCRIPTION
VALTREX (valacyclovir hydrochloride) is the hydrochloride salt of the L‑valyl ester of the antiviral drug acyclovir.
VALTREX tablets are for oral administration. Each tablet contains 556.2 mg or 1.112 grams of valacyclovir hydrochloride equivalent to 500 mg or 1 gram of valacyclovir, respectively, and the inactive ingredients carnauba wax, colloidal silicon dioxide, crospovidone, FD&C Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide. The blue, film‑coated tablets are printed with edible white ink.
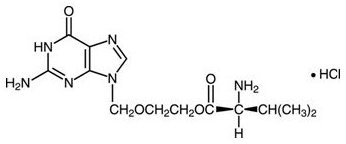
The chemical name of valacyclovir hydrochloride is L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester, monohydrochloride. It has the following structural formula:
Valacyclovir hydrochloride is a white to off-white powder with the molecular formula C13H20N6O4•HCl and a molecular weight of 360.80. The maximum solubility in water at 25°C is 174 mg/mL. The pkas for valacyclovir hydrochloride are 1.90, 7.47, and 9.43.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Valacyclovir is an antiviral drug active against α-herpes viruses [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetics of valacyclovir and acyclovir after oral administration of VALTREX have been investigated in 14 volunteer trials involving 283 adults and in 3 trials involving 112 pediatric subjects aged 1 month to less than 12 years.
Pharmacokinetics in Adults
Absorption and Bioavailability: After oral administration, valacyclovir hydrochloride is rapidly absorbed from the gastrointestinal tract and nearly completely converted to acyclovir and L-valine by first-pass intestinal and/or hepatic metabolism.
The absolute bioavailability of acyclovir after administration of VALTREX is 54.5% ± 9.1% as determined following a 1‑gram oral dose of VALTREX and a 350-mg intravenous acyclovir dose to 12 healthy volunteers. Acyclovir bioavailability from the administration of VALTREX is not altered by administration with food (30 minutes after an 873 Kcal breakfast, which included 51 grams of fat).
Acyclovir pharmacokinetic parameter estimates following administration of VALTREX to healthy adult volunteers are presented in Table 3. There was a less than dose-proportional increase in acyclovir maximum concentration (Cmax) and area under the acyclovir concentration-time curve (AUC) after single-dose and multiple-dose administration (4 times daily) of VALTREX from doses between 250 mg to 1 gram.
There is no accumulation of acyclovir after the administration of valacyclovir at the recommended dosage regimens in adults with normal renal function.
Table 3. Mean (±SD) Plasma Acyclovir Pharmacokinetic Parameters Following Administration of VALTREX to Healthy Adult Volunteers a Administered 4 times daily for 11 days.
ND = not done.Dose
Single‑Dose Administration
(N = 8)
Multiple‑Dose Administrationa
(N = 24, 8 per treatment arm)
Cmax (±SD)
(mcg/mL)
AUC (±SD) (h●mcg/mL)
Cmax (±SD) (mcg/mL)
AUC (±SD) (h●mcg/mL)
100 mg
0.83 (±0.14)
2.28 (±0.40)
ND
ND
250 mg
2.15 (±0.50)
5.76 (±0.60)
2.11 (±0.33)
5.66 (±1.09)
500 mg
3.28 (±0.83)
11.59 (±1.79)
3.69 (±0.87)
9.88 (±2.01)
750 mg
4.17 (±1.14)
14.11 (±3.54)
ND
ND
1,000 mg
5.65 (±2.37)
19.52 (±6.04)
4.96 (±0.64)
15.70 (±2.27)
Distribution: The binding of valacyclovir to human plasma proteins ranges from 13.5% to 17.9%. The binding of acyclovir to human plasma proteins ranges from 9% to 33%.
Metabolism: Valacyclovir is converted to acyclovir and L-valine by first-pass intestinal and/or hepatic metabolism. Acyclovir is converted to a small extent to inactive metabolites by aldehyde oxidase and by alcohol and aldehyde dehydrogenase. Neither valacyclovir nor acyclovir is metabolized by cytochrome P450 enzymes. Plasma concentrations of unconverted valacyclovir are low and transient, generally becoming non-quantifiable by 3 hours after administration. Peak plasma valacyclovir concentrations are generally less than 0.5 mcg/mL at all doses. After single-dose administration of 1 gram of VALTREX, average plasma valacyclovir concentrations observed were 0.5, 0.4, and 0.8 mcg/mL in subjects with hepatic dysfunction, renal insufficiency, and in healthy subjects who received concomitant cimetidine and probenecid, respectively.
Elimination: The pharmacokinetic disposition of acyclovir delivered by valacyclovir is consistent with previous experience from intravenous and oral acyclovir. Following the oral administration of a single 1-gram dose of radiolabeled valacyclovir to 4 healthy subjects, 46% and 47% of administered radioactivity was recovered in urine and feces, respectively, over 96 hours. Acyclovir accounted for 89% of the radioactivity excreted in the urine. Renal clearance of acyclovir following the administration of a single 1-gram dose of VALTREX to 12 healthy subjects was approximately 255 ± 86 mL/min which represents 42% of total acyclovir apparent plasma clearance.
The plasma elimination half‑life of acyclovir typically averaged 2.5 to 3.3 hours in all trials of VALTREX in subjects with normal renal function.
Specific Populations
Patients with Renal Impairment: Reduction in dosage is recommended in patients with renal impairment [see Dosage and Administration (2.4), Use in Specific Populations (8.5, 8.6)].
Following administration of VALTREX to subjects with ESRD, the average acyclovir half‑life is approximately 14 hours. During hemodialysis, the acyclovir half‑life is approximately 4 hours. Approximately one‑third of acyclovir in the body is removed by dialysis during a 4‑hour hemodialysis session. Apparent plasma clearance of acyclovir in subjects on dialysis was 86.3 ± 21.3 mL/min/1.73 m2 compared with 679.16 ± 162.76 mL/min/1.73 m2 in healthy subjects.
Patients with Hepatic Impairment: Administration of VALTREX to subjects with moderate (biopsy‑proven cirrhosis) or severe (with and without ascites and biopsy-proven cirrhosis) liver disease indicated that the rate but not the extent of conversion of valacyclovir to acyclovir is reduced, and the acyclovir half‑life is not affected. Dosage modification is not recommended for patients with cirrhosis.
Patients with HIV-1 Disease: In 9 subjects with HIV-1 disease and CD4+ cell counts less than 150 cells/mm3 who received VALTREX at a dosage of 1 gram 4 times daily for 30 days, the pharmacokinetics of valacyclovir and acyclovir were not different from that observed in healthy subjects.
Geriatric Patients: After single-dose administration of 1 gram of VALTREX in healthy geriatric subjects, the half‑life of acyclovir was 3.11 ± 0.51 hours compared with 2.91 ± 0.63 hours in healthy younger adult subjects. The pharmacokinetics of acyclovir following single- and multiple‑dose oral administration of VALTREX in geriatric subjects varied with renal function. Dose reduction may be required in geriatric patients, depending on the underlying renal status of the patient [see Dosage and Administration (2.4), Use in Specific Populations (8.5, 8.6)].
Pediatric Patients: Acyclovir pharmacokinetics have been evaluated in a total of 98 pediatric subjects (aged 1 month to less than 12 years) following administration of the first dose of an extemporaneous oral suspension of valacyclovir [see Adverse Reactions (6.2), Use in Specific Populations (8.4)]. Acyclovir pharmacokinetic parameter estimates following a 20‑mg/kg dose are provided in Table 4.
Table 4. Mean (±SD) Plasma Acyclovir Pharmacokinetic Parameter Estimates Following First-Dose Administration of 20 mg/kg Valacyclovir Oral Suspension to Pediatric Subjects vs. 1-Gram Single Dose of VALTREX to Adults a Historical estimates using pediatric pharmacokinetic sampling schedule. Parameter
Pediatric Subjects
(20 mg/kg Oral Suspension)
Adults
1‑gram Solid Dose of VALTREXa
(n = 15)
1 -<2 year
(n = 6)
2 -<6 year
(n = 12)
6 -<12 year
(n = 8)
AUC (mcg•h/mL)
14.4 (±6.26)
10.1 (±3.35)
13.1 (±3.43)
17.2 (±3.10)
Cmax (mcg/mL)
4.03 (±1.37)
3.75 (±1.14)
4.71 (±1.20)
4.72 (±1.37)
Drug Interaction Studies
When VALTREX is coadministered with antacids, cimetidine and/or probenecid, digoxin, or thiazide diuretics in patients with normal renal function, the effects are not considered to be of clinical significance (see below). Therefore, when VALTREX is coadministered with these drugs in patients with normal renal function, no dosage adjustment is recommended.
Antacids: The pharmacokinetics of acyclovir after a single dose of VALTREX (1 gram) were unchanged by coadministration of a single dose of antacids (Al3+ or Mg++).
Cimetidine: Acyclovir Cmax and AUC following a single dose of VALTREX (1 gram) increased by 8% and 32%, respectively, after a single dose of cimetidine (800 mg).
Cimetidine plus Probenecid: Acyclovir Cmax and AUC following a single dose of VALTREX (1 gram) increased by 30% and 78%, respectively, after a combination of cimetidine and probenecid, primarily due to a reduction in renal clearance of acyclovir.
Digoxin: The pharmacokinetics of digoxin were not affected by coadministration of VALTREX 1 gram 3 times daily, and the pharmacokinetics of acyclovir after a single dose of VALTREX (1 gram) was unchanged by coadministration of digoxin (2 doses of 0.75 mg).
Probenecid: Acyclovir Cmax and AUC following a single dose of VALTREX (1 gram) increased by 22% and 49%, respectively, after probenecid (1 gram).
Thiazide Diuretics: The pharmacokinetics of acyclovir after a single dose of VALTREX (1 gram) were unchanged by coadministration of multiple doses of thiazide diuretics.
12.4 Microbiology
Mechanism of Action
Valacyclovir is a deoxynucleoside analogue DNA polymerase inhibitor. Valacyclovir hydrochloride is rapidly converted to acyclovir, which has demonstrated antiviral activity against HSV types 1 (HSV‑1) and 2 (HSV‑2) and VZV both in cell culture and in vivo.
Acyclovir is a synthetic purine deoxynucleoside that is phosphorylated intracellularly by the viral encoded thymidine kinase (TK; pUL23) of HSV or VZV into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In biochemical assays, acyclovir triphosphate inhibits replication of α-herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared with VZV is due to its more efficient phosphorylation by the viral TK.
Antiviral Activity
The quantitative relationship between the cell culture susceptibility of herpesviruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (EC50), vary greatly depending upon a number of factors. Using plaque‑reduction assays, the EC50 values against herpes simplex virus isolates range from 0.09 to 60 microM (0.02 to 13.5 mcg/mL) for HSV‑1 and from 0.04 to 44 microM (0.01 to 9.9 mcg/mL) for HSV‑2. The EC50 values for acyclovir against most laboratory strains and clinical isolates of VZV range from 0.53 to 48 microM (0.12 to 10.8 mcg/mL). Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean EC50 value of 6 microM (1.35 mcg/mL).
Resistance
In Cell Culture: Acyclovir-resistant HSV-1, HSV-2, and VZV strains were isolated in cell culture. Acyclovir-resistant HSV and VZV resulted from mutations in the viral thymidine kinase (TK, pUL23) and DNA polymerase (POL; pUL30) genes. Frameshifts were commonly isolated and result in premature truncation of the HSV TK product with consequent decreased susceptibility to acyclovir. Mutations in the viral TK gene may lead to complete loss of TK activity (TK negative), reduced levels of TK activity (TK partial), or alteration in the ability of viral TK to phosphorylate the drug without an equivalent loss in the ability to phosphorylate thymidine (TK altered). In cell culture, acyclovir resistance-associated substitutions in TK of HSV-1 and HSV-2 were observed (Table 5).
Table 5. Summary of Acyclovir Resistance-Associated Amino Acid Substitutions in Cell Culture Virus
Gene
Substitution
HSV-1
TK
P5A, H7Q, L50V, G56V, G59R/V/W/A, G61A/V, K62I/N, T63A, E83K, P84L/S, R89W, D116N, P131S, P155R, F161I/C, R163H/P, A167V, P173L, R176Q/W, Q185R, A189L/V, G200S, G206R, R216S, R220H, L227F, Y239S, T245M, Q261stop, R281stop, T287M, M322K, C336Y, V348A
HSV-2
TK
L69P, C172R, A175V, T288M
HSV-1
POL
D368A, Y557S, E597D, V621S, L702H, A719V, S742N, N815S, V817M, Y818C, G841C/S
HSV-2
POL
No substitutions detected
HSV-Infected Patients: Clinical HSV-1 and HSV-2 isolates obtained from patients who failed treatment for their α-herpes virus infections were evaluated for genotypic changes in the TK and POL genes and for phenotypic resistance to acyclovir (Table 6). HSV isolates with frameshift mutations and resistance-associated substitutions in TK and POL were identified. The listing of substitutions in the HSV TK and POL leading to decreased susceptibility to acyclovir is not all inclusive and additional changes will likely be identified in HSV variants isolated from patients who fail acyclovir-containing regimens. The possibility of viral resistance to acyclovir should be considered in patients who fail to respond or experience recurrent viral shedding during therapy.
Table 6. Summary of Acyclovir Resistance-Associated Amino Acid Substitutions Observed in Treated Patients Note: Many additional pathways to acyclovir resistance likely exist. Virus
Gene
Substitution
HSV-1
TK
G6C, R32H, R41H, R51W, Y53C/D/H, Y53stop, D55N, G56D/E/S, P57H, G58N/R, G59R, G61A/E/W, K62N, T63I, Q67stop, S74stop, Y80N, E83K, P84L, Y87H, E95stop, T103P, Q104H, Q104stop, H105P, M121K/L/R, Q125N, M128L, G129D, I143V, A156V, D162A/H/N, R163G/H, L170P, Y172C, P173L/R, A174P, A175V, R176Q/W, R176stop, L178R, S181N, A186P, V187M, A189V, V192A, G200C/D/S, T201P, T202A, V204G, A207P, L208F/H, R216C/H, R220C/H, R221C/H, R222C/H, E226K, D229H, L242P, T245M/P, L249P, Q250stop, C251G, E257K, Q261R, A265T, R281stop, T287M, L288stop, L291R, L297S, L315S, L327R, C336Y, C336stop, Q342stop, T354P, L364P, A365T
HSV-2
TK
G25A, R34C, G39E, R51W, Y53N/D, G59P, G61A/E/W, S66P, A72S, D78N, P85S, R86P, A94V, L98stop, N100H, I101S, Q103stop, Q105P, A125T, T131P, Y133F, D137stop, F140L, L158P, S169P, R177W, S182N, M183Istop, V192M, G201D, R217H, R221C/H, Q222stop, R223H, D229stop, Y239stop, D231N, L263stop, R271V, P272S, D273R, T287M, C337Y
HSV-1
POL
K532T, S559L, Q570R, L583V, A605V, V621S, A657T, D672N, V715G, A719T/V, S724N, F733C, E771Q, S775N, L778M, E798K, V813M, N815S, G841S, R842S, I890M, V958L, H1228D
HSV-2
POL
E250Q, D307N, K533E, A606V, C625R, R628C, E678G, A724V, S725G, S729N, I731F, Q732R, D785N, M789K/T, V818A, N820S, Y823C, Q829R, T843A, M910T, D912N/V, A915V, F923L, T934A, R964H
Cross-Resistance
Cross-resistance has been observed among HSV isolates carrying frameshift mutations and resistance-associated substitutions, which confer reduced susceptibility to penciclovir (PCV), famciclovir (FCV), and foscarnet (FOS) (Table 7).
Table 7. Summary of Acyclovir Resistance-Associated Amino Acid Substitutions Conferring Cross-Resistance to PCV, FCV or FOS Cross-Resistant Drug
Virus/Gene
Substitution
PCV/FCV
HSV-1 TK
G6C, R32H, R51W, Y53C/H/N, H58N, G61A, S74stop, E83K, P84L, T103P, Q104stop, D116N, M121R, I143V, P155R, R163G/H, A167V, L170P, Y172C, P173L, A174P, R176Q/W, Q185R, A186P, A189L/V, G200D/S, G206R, L208H, R216C, R220H, R222C/H, Y239S, T245M, Q250stop, Q261stop, R281stop, T287M, L315S, M322K, C336Y, V348A
HSV-1 POL
A657T, D672N, V715G, A719V, S724N, E798K, N815S, G841C/S
HSV-2 TK
G39E, R51W, Y53N, R86P, Y133F, R177W, R221H, T288M
HSV-2 POL
K533E, A606V, C625R, R628C, S729N, Q732R, M789K/T, V818A, N820S, F923L, T934A
FOS
HSV-1 POL
D368A, A605V, D672N, L702H, V715G, A719T/V, S724N, L778M, E798K, V813M, N815S, V817M, G841C/S, I890M
HSV-2 POL
K533E, A606V, C625R, R628C, A724V, S725G, S729N, I731F, Q732R, M789K/T, V818A, Y823C, D912V, F923L, T934A, R964H
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The data presented below include references to the steady‑state acyclovir AUC observed in humans treated with 1 gram of VALTREX given orally 3 times a day to treat herpes zoster. Plasma drug concentrations in animal studies are expressed as multiples of human exposure to acyclovir [see Clinical Pharmacology (12.3)].
Carcinogenesis
Valacyclovir was noncarcinogenic in lifetime carcinogenicity bioassays at single daily doses (gavage) of valacyclovir giving plasma acyclovir concentrations equivalent to human levels in the mouse bioassay and 1.4 to 2.3 times human levels in the rat bioassay. There was no significant difference in the incidence of tumors between treated and control animals, nor did valacyclovir shorten the latency of tumors.
Mutagenesis
Valacyclovir was tested in 5 genetic toxicity assays. An Ames assay was negative in the absence or presence of metabolic activation. Also negative were an in vitro cytogenetic study with human lymphocytes and a rat cytogenetic study.
In the mouse lymphoma assay, valacyclovir was not mutagenic in the absence of metabolic activation. In the presence of metabolic activation (76% to 88% conversion to acyclovir), valacyclovir was mutagenic.
Valacyclovir was mutagenic in a mouse micronucleus assay.
Impairment of Fertility
Valacyclovir did not impair fertility or reproduction in male or female rats at acyclovir exposures (AUC) 6 times higher than in humans given the MRHD. Testicular atrophy occurred in male rats (orally dosed for 97 days at 18 times the MRHD) and was reversible.
-
14 CLINICAL STUDIES
14.1 Cold Sores (Herpes Labialis)
Two double‑blind, placebo‑controlled clinical trials were conducted in 1,856 healthy adults and adolescents (aged greater than or equal to 12 years) with a history of recurrent cold sores. Subjects self‑initiated therapy at the earliest symptoms and prior to any signs of a cold sore. The majority of subjects initiated treatment within 2 hours of onset of symptoms. Subjects were randomized to VALTREX 2 grams twice daily on Day 1 followed by placebo on Day 2, VALTREX 2 grams twice daily on Day 1 followed by 1 gram twice daily on Day 2, or placebo on Days 1 and 2.
The mean duration of cold sore episodes was about 1 day shorter in treated subjects as compared with placebo. The 2-day regimen did not offer additional benefit over the 1-day regimen.
No significant difference was observed between subjects receiving VALTREX or placebo in the prevention of progression of cold sore lesions beyond the papular stage.
14.2 Genital Herpes Infections
Initial Episode
Six hundred forty-three immunocompetent adults with first-episode genital herpes who presented within 72 hours of symptom onset were randomized in a double-blind trial to receive 10 days of VALTREX 1 gram twice daily (n = 323) or oral acyclovir 200 mg 5 times a day (n = 320). For both treatment groups the median time to lesion healing was 9 days, the median time to cessation of pain was 5 days, and the median time to cessation of viral shedding was 3 days.
Recurrent Episodes
Three double-blind trials (2 of them placebo-controlled) in immunocompetent adults with recurrent genital herpes were conducted. Subjects self-initiated therapy within 24 hours of the first sign or symptom of a recurrent genital herpes episode.
In 1 trial, subjects were randomized to receive 5 days of treatment with either VALTREX 500 mg twice daily (n = 360) or placebo (n = 259). The median time to lesion healing was 4 days in the group receiving VALTREX 500 mg versus 6 days in the placebo group, and the median time to cessation of viral shedding in subjects with at least 1 positive culture (42% of the overall trial population) was 2 days in the group receiving VALTREX 500 mg versus 4 days in the placebo group. The median time to cessation of pain was 3 days in the group receiving VALTREX 500 mg versus 4 days in the placebo group. Results supporting efficacy were replicated in a second trial.
In a third trial, subjects were randomized to receive VALTREX 500 mg twice daily for 5 days (n = 398) or VALTREX 500 mg twice daily for 3 days (and matching placebo twice daily for 2 additional days) (n = 402). The median time to lesion healing was about 4½ days in both treatment groups. The median time to cessation of pain was about 3 days in both treatment groups.
Suppressive Therapy
Two clinical trials were conducted, one in immunocompetent adults and one in HIV-1–infected adults.
A double‑blind, 12‑month, placebo‑ and active‑controlled trial enrolled immunocompetent adults with a history of 6 or more recurrences per year. Outcomes for the overall trial population are shown in Table 8.
Table 8. Recurrence Rates in Immunocompetent Adults at 6 and 12 Months a Includes lost to follow-up, discontinuations due to adverse events, and consent withdrawn. Outcome
6 Months
12 Months
VALTREX
1 gram
Once Daily
(n = 269)
Oral Acyclovir
400 mg
Twice Daily
(n = 267)
Placebo
(n = 134)
VALTREX
1 gram Once Daily
(n = 269)
Oral Acyclovir
400 mg
Twice Daily
(n = 267)
Placebo
(n = 134)
Recurrence free
55%
54%
7%
34%
34%
4%
Recurrences
35%
36%
83%
46%
46%
85%
Unknowna
10%
10%
10%
19%
19%
10%
Subjects with 9 or fewer recurrences per year showed comparable results with VALTREX 500 mg once daily.
In a second trial, 293 HIV‑1–infected adults on stable antiretroviral therapy with a history of 4 or more recurrences of ano‑genital herpes per year were randomized to receive either VALTREX 500 mg twice daily (n = 194) or matching placebo (n = 99) for 6 months. The median duration of recurrent genital herpes in enrolled subjects was 8 years, and the median number of recurrences in the year prior to enrollment was 5. Overall, the median pretrial HIV‑1 RNA was 2.6 log10 copies/mL. Among subjects who received VALTREX, the pretrial median CD4+ cell count was 336 cells/mm3; 11% had less than 100 cells/mm3, 16% had 100 to 199 cells/mm3, 42% had 200 to 499 cells/mm3, and 31% had greater than or equal to 500 cells/mm3. Outcomes for the overall trial population are shown in Table 9.
Table 9. Recurrence Rates in HIV-1–Infected Adults at 6 Months a Includes lost to follow-up, discontinuations due to adverse events, and consent withdrawn. Outcome
VALTREX
500 mg Twice Daily
(n = 194)
Placebo
(n = 99)
Recurrence free
65%
26%
Recurrences
17%
57%
Unknowna
18%
17%
Reduction of Transmission of Genital Herpes
A double‑blind, placebo‑controlled trial to assess transmission of genital herpes was conducted in 1,484 monogamous, heterosexual, immunocompetent adult couples. The couples were discordant for HSV‑2 infection. The source partner had a history of 9 or fewer genital herpes episodes per year. Both partners were counseled on safer sex practices and were advised to use condoms throughout the trial period. Source partners were randomized to treatment with either VALTREX 500 mg once daily or placebo once daily for 8 months. The primary efficacy endpoint was symptomatic acquisition of HSV‑2 in susceptible partners. Overall HSV‑2 acquisition was defined as symptomatic HSV‑2 acquisition and/or HSV‑2 seroconversion in susceptible partners. The efficacy results are summarized in Table 10.
Table 10. Percentage of Susceptible Partners Who Acquired HSV-2 Defined by the Primary and Selected Secondary Endpoints a Results show reductions in risk of 75% (symptomatic HSV‑2 acquisition), 50% (HSV‑2 seroconversion), and 48% (overall HSV‑2 acquisition) with VALTREX versus placebo. Individual results may vary based on consistency of safer sex practices. Endpoint
VALTREXa
(n = 743)
Placebo
(n = 741)
Symptomatic HSV‑2 acquisition
4 (0.5%)
16 (2.2%)
HSV‑2 seroconversion
12 (1.6%)
24 (3.2%)
Overall HSV‑2 acquisition
14 (1.9%)
27 (3.6%)
14.3 Herpes Zoster
Two randomized, double‑blind clinical trials in immunocompetent adults with localized herpes zoster were conducted. VALTREX was compared with placebo in subjects aged less than 50 years and with oral acyclovir in subjects aged greater than 50 years. All subjects were treated within 72 hours of appearance of zoster rash. In subjects aged less than 50 years, the median time to cessation of new lesion formation was 2 days for those treated with VALTREX compared with 3 days for those treated with placebo. In subjects aged greater than 50 years, the median time to cessation of new lesions was 3 days in subjects treated with either VALTREX or oral acyclovir. In subjects aged less than 50 years, no difference was found with respect to the duration of pain after healing (post‑herpetic neuralgia) between the recipients of VALTREX and placebo. In subjects aged greater than 50 years, among the 83% who reported pain after healing (post‑herpetic neuralgia), the median duration of pain after healing (95% CI) in days was: 40 (31, 51), 43 (36, 55), and 59 (41, 77) for 7‑day VALTREX, 14‑day VALTREX, and 7‑day oral acyclovir, respectively.
14.4 Chickenpox
The use of VALTREX for treatment of chickenpox in pediatric subjects aged 2 to less than 18 years is based on single‑dose pharmacokinetic and multiple‑dose safety data from an open‑label trial with valacyclovir and supported by safety and extrapolated efficacy data from 3 randomized, double‑blind, placebo‑controlled trials evaluating oral acyclovir in pediatric subjects.
The single‑dose pharmacokinetic and multiple‑dose safety trial enrolled 27 pediatric subjects aged 1 to less than 12 years with clinically suspected VZV infection. Each subject was dosed with valacyclovir oral suspension, 20 mg/kg 3 times daily for 5 days. Acyclovir systemic exposures in pediatric subjects following valacyclovir oral suspension were compared with historical acyclovir systemic exposures in immunocompetent adults receiving the solid oral dosage form of valacyclovir or acyclovir for the treatment of herpes zoster. The mean projected daily acyclovir exposures in pediatric subjects across all age‑groups (1 to less than 12 years) were lower (Cmax: ↓13%, AUC: ↓30%) than the mean daily historical exposures in adults receiving valacyclovir 1 gram 3 times daily, but were higher (daily AUC: ↑50%) than the mean daily historical exposures in adults receiving acyclovir 800 mg 5 times daily. The projected daily exposures in pediatric subjects were greater (daily AUC approximately 100% greater) than the exposures seen in immunocompetent pediatric subjects receiving acyclovir 20 mg/kg 4 times daily for the treatment of chickenpox. Based on the pharmacokinetic and safety data from this trial and the safety and extrapolated efficacy data from the acyclovir trials, oral valacyclovir 20 mg/kg 3 times a day for 5 days (not to exceed 1 gram 3 times daily) is recommended for the treatment of chickenpox in pediatric patients aged 2 to less than 18 years. Because the efficacy and safety of acyclovir for the treatment of chickenpox in children aged less than 2 years have not been established, efficacy data cannot be extrapolated to support valacyclovir treatment in children aged less than 2 years with chickenpox. Valacyclovir is also not recommended for the treatment of herpes zoster in children because safety data up to 7 days’ duration are not available [see Use in Specific Populations (8.4)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VALTREX tablets (blue, film‑coated, capsule‑shaped tablets printed with “VALTREX 500 mg”) containing 556.2 mg of valacyclovir hydrochloride equivalent to 500 mg valacyclovir.
Bottle of 30 (NDC 0173-0933-08).
Bottle of 90 (NDC 0173-0933-10).
VALTREX tablets (blue, film‑coated, capsule‑shaped tablets, with a partial scorebar on both sides, printed with “VALTREX 1 gram”) containing 1.112 grams of valacyclovir hydrochloride equivalent to 1 gram of valacyclovir.
Bottle of 30 (NDC 0173-0565-04).
Bottle of 90 (NDC 0173-0565-10).
Storage:
Store at 15°C to 25°C (59°F to 77°F). Dispense in a well-closed container as defined in the USP.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Importance of Adequate Hydration
Patients should be advised to maintain adequate hydration.
Missed Dose
Instruct patients that if they miss a dose of VALTREX, to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose.
Cold Sores (Herpes Labialis)
Patients should be advised to initiate treatment at the earliest symptom of a cold sore (e.g., tingling, itching, or burning). There are no data on the effectiveness of treatment initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer). Patients should be instructed that treatment for cold sores should not exceed 1 day (2 doses) and that their doses should be taken about 12 hours apart. Patients should be informed that VALTREX is not a cure for cold sores.
Genital Herpes
Patients should be informed that VALTREX is not a cure for genital herpes. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes is frequently transmitted in the absence of symptoms through asymptomatic viral shedding. Therefore, patients should be counseled to use safer sex practices in combination with suppressive therapy with VALTREX. Sex partners of infected persons should be advised that they might be infected even if they have no symptoms. Type‑specific serologic testing of asymptomatic partners of persons with genital herpes can determine whether risk for HSV‑2 acquisition exists.
VALTREX has not been shown to reduce transmission of sexually transmitted infections other than HSV‑2.
If medical management of a genital herpes recurrence is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
There are no data on the effectiveness of treatment initiated more than 72 hours after the onset of signs and symptoms of a first episode of genital herpes or more than 24 hours after the onset of signs and symptoms of a recurrent episode.
There are no data on the safety or effectiveness of chronic suppressive therapy of more than 1 year’s duration in otherwise healthy patients. There are no data on the safety or effectiveness of chronic suppressive therapy of more than 6 months’ duration in HIV-1−infected patients.
Herpes Zoster
There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Chickenpox
Patients should be advised to initiate treatment at the earliest sign or symptom of chickenpox.
Trademarks are owned by or licensed to the GSK group of companies.
Distributed by:
GlaxoSmithKline
Durham, NC 27701
©2022 GSK group of companies or its licensor.
VTX: 11PI
PHARMACIST‑DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
VALTREX (VAL-trex)
(valacyclovir)
tablets
What is VALTREX?
VALTREX is a prescription medicine used in adults:
- •
- to treat cold sores (herpes labialis).
- •
- to treat or control genital herpes outbreaks in adults with normal immune systems.
- •
- to control genital herpes outbreaks in adults with human immunodeficiency virus-1 (HIV-1).
- •
- with safer sex practices to lower the chance of spreading genital herpes to others, in adults with normal immune systems. Even with safer sex practices, it is still possible to spread genital herpes.
- o
- Do not have sexual contact with your partner when you have any symptom or outbreak of genital herpes.
- o
- Use a condom made of latex or polyurethane whenever you have sexual contact.
- o
- Ask your healthcare provider for more information about safer sex practices.
- •
- to treat shingles (herpes zoster) in adults with normal immune systems.
VALTREX is used in children to treat:
- •
- cold sores in children 12 years of age and older.
- •
- chickenpox in children with normal immune systems 2 years of age to less than 18 years of age.
VALTREX does not cure cold sores, chickenpox, shingles, or genital herpes.
- •
- It is not known if VALTREX is safe and effective in people with weakened immune systems, other than for control of outbreaks of genital herpes in people with HIV-1.
- •
- It is not known if VALTREX is safe and effective in people 18 years of age and older with chickenpox.
- •
- It is not known if VALTREX is safe and effective in children:
- o
- less than 12 years of age with cold sores
- o
- less than 2 years of age with chickenpox
- o
- less than 18 years of age with genital herpes or shingles
Do not take VALTREX if you are allergic to valacyclovir, acyclovir, or any of the ingredients in VALTREX. See the end of this leaflet for a complete list of ingredients in VALTREX.
Before you take VALTREX, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have had a bone marrow transplant or kidney transplant, or if you have advanced HIV-1 infection or acquired immune deficiency syndrome (AIDS).
- •
- have kidney problems, including if you receive dialysis.
- •
- are pregnant or plan to become pregnant. It is not known if VALTREX will harm your unborn baby. You and your healthcare provider will decide if you will take VALTREX if you are pregnant.
- •
- are breastfeeding or plan to breastfeed. VALTREX may pass into your breastmilk. Talk with your healthcare provider about the best way to feed your child if you take VALTREX.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take VALTREX?
- •
- Take VALTREX exactly as your healthcare provider tells you to take it.
- •
- Your dose of VALTREX and length of treatment will depend on the type of infection that you have and any other medical problems that you have.
- •
- Do not stop VALTREX or change your treatment without talking to your healthcare provider.
- •
- Take VALTREX with or without food.
- •
- Tell your healthcare provider if your child cannot swallow VALTREX tablets. Your healthcare provider can prescribe VALTREX as an oral suspension for your child.
- •
- If you are taking VALTREX to treat outbreaks of cold sores, chickenpox, shingles, or genital herpes, take VALTREX as soon as you have the first symptoms of infection such as tingling, itching, or burning, or when the sore appears.
- •
- It is important for you to stay well hydrated during treatment with VALTREX. Be sure to drink plenty of fluids during this time.
- •
- If you miss a dose of VALTREX, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose. Take the next dose at your regular time. Do not take 2 doses at the same time or take more VALTREX than prescribed.
- •
- If you take too much VALTREX, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of VALTREX?
VALTREX can cause serious side effects including:
- •
- Thrombotic Thrombocytopenic Purpura (TTP) and Hemolytic Uremic Syndrome (HUS). TTP and HUS have happened in people with weakened immune systems taking VALTREX and have led to death. TTP and HUS are disorders that can cause small blood clots to form throughout the body and decrease blood flow to body organs such as the brain, heart, and kidneys. Your healthcare provider will stop treatment with VALTREX if you have signs or symptoms of TTP and HUS.
- •
- kidney failure.
- •
- nervous system problems. Tell your healthcare provider right away if you get any of these signs or symptoms of nervous system problems during treatment with VALTREX:
- o
- aggressive behavior
- o
- unsteady movement
- o
- shaky movements
- o
- confusion
- o
- speech problems
- o
- hallucinations (seeing or hearing things that are really not there)
- o
- seizures
- o
- coma
Elderly people are more likely to get certain side effects. Talk to your healthcare provider if this is a concern for you.
The most common side effects of VALTREX in adults include:
- •
- headache
- •
- nausea
- •
- stomach (abdominal) pain
The most common side effect of VALTREX in children less than 18 years of age is headache.
These are not all the possible side effects of VALTREX.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
How should I store VALTREX?
- •
- Store VALTREX tablets at 59°F to 77°F (15°C to 25°C).
- •
- Store VALTREX suspension between 36°F to 46°F (2°C to 8°C) in a refrigerator. Throw away (discard) any remaining VALTREX suspension after 28 days.
- •
- Shake VALTREX suspension bottle well before using.
- •
- Keep VALTREX in a tightly closed container.
Keep VALTREX and all medicines out of the reach of children.
General information about the safe and effective use of VALTREX.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VALTREX for a condition for which it was not prescribed. Do not give VALTREX to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about VALTREX that is written for health professionals.
What are the ingredients in VALTREX?
Active ingredient: valacyclovir hydrochloride
Inactive ingredients: carnauba wax, colloidal silicon dioxide, crospovidone, FD&C Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide.
Distributed by:
GlaxoSmithKline
Durham, NC 27701
Trademarks are owned by or licensed to the GSK group of companies.
©2022 GSK group of companies or its licensor.
VTX:9PIL
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 11/2022
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0173-0933-08
VALTREX
(valacyclovir) TABLETS
500 mg
30 Tablets
Rx only
GSK
Equivalent to 556.2 mg valacyclovir hydrochloride per tablet.
See prescribing information for dosage information.
Keep out of reach of children.
Store at 15o to 25oC (59o to 77oF).
Dispense in a well-closed container as defined in the USP.
Do not accept if membrane seal under cap is missing or broken.
Distributed by:
GlaxoSmithKline
Durham, NC 27701
Made in India
Trademarks are owned by or licensed to the GSK group companies.
©2023 GSK group of companies or its licensor.
Rev. 3/23
62000000082733
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0173-0565-04
VALTREX
(valacyclovir) TABLETS
1 gram
30 Tablets
Rx only
GSK
Equivalent to 1.112 grams valacyclovir hydrochloride per tablet.
See prescribing information for dosage information.
Keep out of reach of children.
Store at 15o to 25oC (59o to 77oF). Dispense in a well-closed container as defined in the USP.
Do not accept if membrane seal under cap is missing or broken.
Distributed by:
GlaxoSmithKline
Durham, NC 27701
Made in India
Trademarks are owned by or licensed to the GSK group companies.
©2023 GSK group of companies or its licensor.
Rev. 3/23
62000000082730
-
INGREDIENTS AND APPEARANCE
VALTREX
valacyclovir hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0173-0933 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALACYCLOVIR HYDROCHLORIDE (UNII: G447S0T1VC) (ACYCLOVIR - UNII:X4HES1O11F) VALACYCLOVIR 500 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BLUE Score no score Shape OVAL (capsule-shaped) Size 18mm Flavor Imprint Code VALTREX;500;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0173-0933-08 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/1995 2 NDC:0173-0933-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020487 08/01/1995 VALTREX
valacyclovir hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0173-0565 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALACYCLOVIR HYDROCHLORIDE (UNII: G447S0T1VC) (ACYCLOVIR - UNII:X4HES1O11F) VALACYCLOVIR 1 g Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BLUE Score 2 pieces Shape OVAL (capsule-shaped) Size 23mm Flavor Imprint Code VALTREX;1;gram Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0173-0565-04 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/12/2002 2 NDC:0173-0565-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/12/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020487 08/12/2002 Labeler - GlaxoSmithKline LLC (167380711)