Label: LIDOCAINE HYDROCHLORIDE solution
- NDC Code(s): 0121-0950-03, 0121-4950-15, 0121-4950-40
- Packager: PAI Holdings, LLC dba PAI Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONWARNING: Life-threatening and fatal events in infants and young children - Postmarketing cases of seizures, cardiopulmonary arrest, and death in patients under the age of 3 years have been ...
-
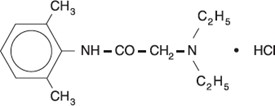
DESCRIPTION:
Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) contains a local anesthetic agent and is administered topically. Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous ...
-
CLINICAL PHARMACOLOGY:
Mechanism of Action - Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic ...
-
INDICATIONS AND USAGE:
Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) is indicated for the production of topical anesthesia of irritated or inflamed mucous membranes of the mouth and pharynx. It is also ...
-
CONTRAINDICATIONS:
Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type, or to other components of the solution.
-
WARNINGS:
EXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS. PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ...
-
PRECAUTIONS:
Information for - Patients - Parents and caregivers should be cautioned about the following: For patients under 3 years of age, special care must be given to accurately measuring the prescribed ...
-
ADVERSE REACTIONS:
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general ...
-
OVERDOSAGE:
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics (see ADVERSE REACTIONS, WARNINGS, and ...
-
DOSAGE AND ADMINISTRATION:
Adult - The maximum recommended single dose of Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) for healthy adults should be such that the dose of lidocaine HCl does not exceed 4.5 ...
-
HOW SUPPLIED:
Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) is a clear colorless viscous liquid with cherry flavor available as: 0121-0950-03: 100 mL polyethylene squeeze ...
-
MANUFACTURED BY:
Greenville, SC 29605 - R05/23
-
PACKAGE LABEL - BOTTLE - 100 mLNDC 0121-0950-03 - Lidocaine Hydrochloride Oral Topical Solution, USP - 2% (Viscous) NOTE: Squeeze bottle to dispense contents. For Oral Use Only - Shake Well Before Using - Rx only - 100 mL
-
PACKAGE LABEL - UNIT DOSE CUP - 15 mLNDC 0121-4950-15 - Delivers 15 mL - Lidocaine Hydrochloride Oral Topical Solution, USP - 2% Viscous - For Oral Use Only - Shake Well Before Using - Package Not Child-Resistant - Rx ONLY - PAI ...
-
INGREDIENTS AND APPEARANCEProduct Information