Opill® Norgestrel tablets 0.075mg
-
Daily Oral Contraceptive
-
FDA APPROVED
-
What you need to know
-
Before you use Opill®, read this information carefully and take Opill® exactly as directed ...
Opill® Norgestrel tablets 0.075mg
Daily Oral Contraceptive
FDA APPROVED
What you need to know
Before you use Opill®, read this information carefully and take Opill® exactly as directed.
Keep this leaflet as it contains important information.
DP30705
What this leaflet covers
- 1.
- What is Opill®?
- 2.
- Is Opill® right for you?
- 3.
- How to take Opill®?
- 4.
- Tips to help you remember to take Opill® on-time
- 5.
- When to talk to a doctor or pharmacist?
- 6.
- What could happen to your periods while taking Opill®?
- 7.
- Other questions you may have
Before you start…
Two things you need to know.
It’s very important to take Opill® every single day at the same time:
- •
- Without breaks
- •
- Even if you have changes in your menstrual period
same time every day
It takes 2 days for Opill® to start working so use a condom (or other barrier method) every time you have sex for the next 2 days (48 hours):
- •
- After you start your first pack of Opill®
- •
- If you take a tablet more than 3 hours late or miss a tablet on 1 or more days
- •
- If you vomit of have severe diarrhea within 4 hours of taking a tablet
OPILL® WILL WORK BEST IF YOU TAKE IT EXACTLY AS DIRECTED
- 1.
-
What is Opill®?
Opill® is a daily oral contraceptive (also called a birth control pill) used by women to prevent pregnancy.
Opill® is NOT an emergency contraceptive (morning after pill). You should not take Opill® to try to prevent pregnancy after unprotected sex because it will not work.
Opill® does NOT protect against HIV/AIDS or other sexually transmitted diseases (STDs). You should use condoms to protect against HIV/AIDS or other STDs.
- 2.
-
Is Opill® right for you?
Most women can use Opill®. However, do not use Opill®:
- •
- If you have or ever had breast cancer, because some breast cancers are sensitive to hormones like the one in Opill®.
- •
- If you know that you are already pregnant or think you may be pregnant.
- •
- Together with another birth control pill, vaginal ring, patch, implant injection or an IUD (intra-uterine device).
- •
- If you are allergic to this product or any of its ingredients, such as the color additive FD&C yellow No. 5 (tartrazine). People allergic to aspirin often have a tartrazine allergy too. Symptoms may include: hives, facial swelling, asthma (wheezing), shock, skin reddening, rash, blisters. If an allergic reaction occurs, stop use and seek medical help right away.
Talk to a doctor before staring Opill® if:
- •
- You currently have vaginal bleeding between your periods and you have not already talked to a doctor, because it could be the sign of a condition that should be treated.
- •
- You have liver tumors or a liver disease.
- •
- You have or ever had any cancer, because some cancers are sensitive to hormones like the one in Opill®.
- 3.
-
How to take Opill®?
-
-
Take 1 tablet at the same time every single day (and no later than 3 hours from the time you took
-
-
your tablet the day before). Never skip your daily tablet.
- •
- Never take a break between packs. When you finish one pack (all 28 tablets), you should start the next pack the following day.
- •
- Take Opill® every single day, even when you have your period, or if you have spotting or bleeding between periods.
- •
- Do not skip tablets even if you do not have sex very often.
To start using Opill®:
- •
- You can start your first pack on any day.
- •
-
If you are switching from another oral contraceptive, vaginal ring, or patch, start taking Opill® the day after you stop the other method.
- •
- You must take your daily tablet at the same time of day every single day.
- •
- You must use a condom (or another barrier method) every time you have sex during the first 2 days (48 hours) because it takes 2 days to start working.
What if I am late taking my tablet?
Less than 3 hours late:
Don’t worry. Take 1 tablet immediately and go back to taking your tablet at your usual time the following day.
More than 3 hours late OR you missed one or more tablets:
- •
- Take 1 tablet immediately, as soon as you remember.
- •
- Then, go back to taking your tablet at your usual time. This means you may take 2 tablets in 1 days.
- For example, if you usually take your tablet at night, but forget and remember in the morning, take 1 tablet when you remember and take 1 tablet again at the usual time that night.
- •
- You must use a condom (or another barrier method) every time you have sex during the 2 days (48 hours) after you restart Opill®, because it takes 2 days to start working again.
- •
-
Take a pregnancy test or talk to a doctor if your period is later after missing any tablets in the last month.
What if I vomit or have severe diarrhea within 4 hours of taking my tablet?
- •
- Use a condom (or another barrier method) every time you have sex for the next 2 days (48 hours) because the medicine may not have been fully absorbed.
- •
- The next day, take your daily tablet at your usual time.
- 4.
-
Tips to help you remember to take Opill® on-time
Choose a convenient time of day. It is best to link this to something you already do at the same time every day. For example, when you wake up or when you brush your teeth.
Set reminders. Consider using your smartphone to set a daily alarm, and put reminders in visible places such as bathroom mirror, phone or coffee machine.
Buy a new pack of Opill®before finishing the pack so you can start the next pack on time.
- 5.
-
When to talk to a doctor or pharmacist?
What if I am taking other medicines or herbal products?
Talk to a doctor or pharmacist if you are taking or start to take any of the following medications, as these may make Opill® less effective:
- •
- Certain drugs to treat
-
- - Seizures (barbiturates, carbamazepine, oxcarbazepine, phenytoin, topiramate, primidone)
-
- - Tuberculosis (rifampin, rifabutin)
-
- - Pulmonary hypertension (bosentan)
-
- - HIV/AIDS (efavirenz)
- •
- St. John’s Wort (or any herbal products containing hypericum perforatum)
Your doctor or pharmacist may advise you to use another form of contraception.
What if I have taken an emergency contraceptive (morning after pill) before starting Opill®?
- •
-
Talk to a doctor or pharmacist if you have taken an emergency contraceptive in the past 5 days.
- •
- Opill® should not be used for 5 days after using the emergency contraceptive ella® which contains ulipristal acetate. This might reduce the ability of both Opill® and ella® to prevent pregnancy. Also, use a condom (or another barrier method) every time you have sex until your next period.
What if I have sudden or severe persistent pain in my lower belly mostly on one side?
Seek medical help right away.
You may have an ectopic pregnancy (a fertilized egg implanted in the wrong place).
While ectopic pregnancy is unlikely if you are taking Opill® as directed, it is a serious risk if it occurs. Severe persistent belly pain may occur for a few reasons and should be assessed right away.
What if I become pregnant while taking Opill®?
- •
-
Stop taking this product and talk to a doctor if you get pregnant while taking Opill®.
- •
- Signs that you may be pregnant might include: missed periods, tender breasts, feeling nauseous, fatigue, and/or needing to urinate urgently or more frequently.
- •
-
Take a pregnancy test or talk to a doctor if you period is late after missing any tablets in the last month, if you have not had a period for 2 months, or if you think you may be pregnant.
What if I get migraines while using Opill®?
If you start having migraines with aura (headaches that start with changes in vision) or your migraine headaches get worse, talk to a doctor but continue taking every day. Some women with migraine may be at increased risk of stroke.
What if I develop yellowing of skin or eyes while using Opill®?
Seek medical help right away if you develop the following rare symptoms: yellowing of the whites of your eyes or skin (especially with fever, tiredness, loss of appetite or dark colored urine). These may be a sign of liver problems which can be a serious medical condition.
- 6.
-
What could happen to your periods while taking Opill®?
What changes in my menstrual period are normal while using Opill®?
Most changes to periods are to be expected.
Continue taking Opill®exactly as directed, even if you have the following changes in your periods:
- •
- Your periods may be less or more frequent, shorter or longer, lighter or heavier than before you started Opill®. You may also have some spotting or bleeding between periods.
- •
- Some women stop having periods while taking Opill®.
- •
-
Take a pregnancy test or talk to a doctor if you period is late after missing any tablets in the last month, if you have not had a period of 2 months, or if you think you may be pregnant. Signs that you may be pregnant are listed in section 5, under « What if I become pregnant while taking Opill.
What changes to my period are NOT expected when using Opill®?
Talk to a doctor AND continue taking this product every day if you experience any of the following:
- •
- You repeatedly have vaginal bleeding that is brought on by sex.
- •
- You start having menstrual periods that last more than 8 days or are unusually heavy.
- 7.
-
Other questions you may have
What type of oral contraceptive is Opill®?
- •
- Opill® is a progestin-only pill (POP)
- •
- Opill® contains the hormone progestin but does not contain estrogen, so it is different than the commonly used combined oral contraceptive, which contains both estrogen and progestin.
Opill® works mainly by thickening your cervical mucus which helps to block sperm from getting to the egg. You must take it every day to prevent pregnancy. In addition, Opill® may prevent your ovaries from releasing eggs.
- •
- Every one of the 28 tablets in your blister pack contains the active ingredient so you must take one tablet every day with no breaks.
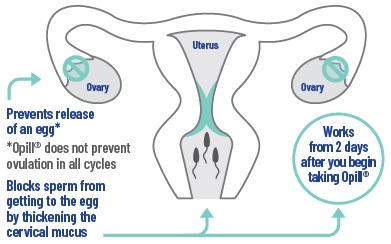
How effective is Opill® at preventing pregnancy?
- •
- As with any birth control method, Opill® does not prevent pregnancy all the time. Opill® will work best if you take it exactly as directed.
- •
- In 8 US clinical trials, approximately 98 out of 100 sexually active women who used Opill® for a year did not become pregnant in that time.
What if I decide I want to get pregnant?
- •
- If you decide you want to become pregnant, simply stop taking Opill®. Opill® will not delay your ability to get pregnant.
Is it okay to use Opill® if I’m breastfeeding?
- •
- Yes. Opill® is safe and effective in breastfeeding women. Small amounts of progestin may pass into the breast milk; however, no adverse effects have been found on either breastfeeding performance or infant health.
What types of side effects may I expect while using Opill®?
- •
- When used as directed, Opill® is safe and effective.
- •
- The most common side effect is changes in menstrual periods (bleeding). See section 6.
- •
- Less common side effects may include headaches, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating.
Routine healthcare
You should continue to see your healthcare provider(s) for routine healthcare visits.
- •
- It is important that you continue to have regular Pap smear tests (cervical screening) while taking Opill®.
- •
- Regular breast screening (mammography) is also recommended.
- •
- If you are worried you may have a Sexually Transmitted Disease (STS) including HIV (AIDS), see your healthcare provider as soon as you can. Many STDs, like HIV, have no symptoms at all. The only way to know for sure that you do not have an STD is to get tested. Only barrier methods (such as condoms) can protect you from STD.
You should tell your healthcare provider(s) that you are taking Opill®.
Regular contraception
There are many different types of contraception available, and you should be able to find the right method for you. The different contraceptive options are listed below from the most effective to the least effective. You can ask your healthcare provider(s) for advice.
Trussell J, Aiken ARA, Micks E, Guthrie KA. Efficacy, safety, and personal considerations. In: Hatcher RA, Nelson AL, Trussell J, Cwiak C, Cason P, Policar MS, Edelman A, Aiken ARA, Marrazzo J, Kowal D, eds. Contraceptive technology. 21st ed. New York, NY: Ayer Company Publishers, Inc., 2018
More about routine healthcare and contraception
For further information on all methods of contraception or screening, go to
- •
- https://www.cdc.gov/reproductivehealth/contraception/index.htm
- •
- https://www.cdc.gov/cancer/breast/basic_info/screening.htm
- •
- https://www.cdc.gov/cancer/cervical/basic_info/screening.htm
What if I still have questions about Opill®?
If you have questions or need more information, call our toll-free number: 1-877-414-6859
Reminder card Front –
Remember to take 1 tablet at the same time every single day*
Take it without breaks
Take is even if you have changes in your menstrual period*
If a tablet is late or missed, use back up contraception for 2 days*
*refer to Drug Facts and consumer information leaflet for more details
Reminder card Back –
Opill®
Norgestrel tablets 0.075mg
I will take my pill every day at: _________AM/PM
A few tips…
- •
- Pair the tablet-taking with a daily task
- •
- Keep the pack visible in the same place
- •
- Put reminders in visible places such as bathroom mirror, phone or coffee machine
- •
- Set an alarm on your smartphone
DP30706
Close