Label: RAMELTEON tablet, film coated
- NDC Code(s): 0832-1250-11, 0832-1250-30
- Packager: Upsher-Smith Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RAMELTEON TABLETS safely and effectively. See full prescribing information for RAMELTEON TABLETS. RAMELTEON tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERamelteon tablets are indicated for the treatment of insomnia characterized by difficulty with sleep onset. The clinical trials performed in support of efficacy were up to six months in duration ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage in Adults - The recommended dose of ramelteon tablets is 8 mg taken within 30 minutes of going to bed. It is recommended that ramelteon tablets not be taken with or immediately after a ...
-
3 DOSAGE FORMS AND STRENGTHSRamelteon tablets are available in an 8 mg strength tablet for oral administration. Ramelteon tablets 8 mg are yellow, round shaped film coated tablets, debossed with "AC 414" on one side and ...
-
4 CONTRAINDICATIONSPatients who develop angioedema after treatment with ramelteon tablets should not be rechallenged with the drug. Patients should not take ramelteon tablets in conjunction with fluvoxamine [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Severe Anaphylactic and Anaphylactoid Reactions - Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections: Severe anaphylactic and anaphylactoid reactions [see Warnings and Precautions (5.1)] Abnormal thinking ...
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on Ramelteon - Fluvoxamine (strong CYP1A2 inhibitor) AUC0-inf for ramelteon increased approximately 190-fold, and the Cmax increased approximately 70- fold upon ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from postmarketing reports with ramelteon use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or ...
-
9 DRUG ABUSE AND DEPENDENCERamelteon tablets are not a controlled substance. Discontinuation of ramelteon in animals or in humans after chronic administration did not produce withdrawal signs. Ramelteon does not appear to ...
-
10 OVERDOSAGEGeneral symptomatic and supportive measures should be used, along with immediate gastric lavage where appropriate. Intravenous fluids should be administered as needed. As in all cases of drug ...
-
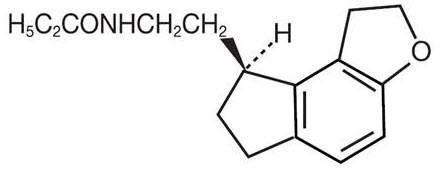
11 DESCRIPTIONRamelteon is an orally active hypnotic chemically designated as (S)-N-[2-(1,6,7,8- tetrahydro-2H-indeno-[5,4-b]furan-8-yl)ethyl]propionamide and containing one chiral center. The compound is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ramelteon is a melatonin receptor agonist with both high affinity for melatonin MT1 and MT2 receptors and relative selectivity over the MT3 receptor. The activity of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Ramelteon was administered to mice and rats at oral doses of 0, 30, 100, 300, or 1,000 mg/kg/day (mice) and 0, 15 ...
-
14 CLINICAL STUDIES14.1 Controlled Clinical Trials - Chronic Insomnia - Three randomized, double-blind trials in subjects with chronic insomnia employing polysomnography (PSG) were provided as objective support ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRamelteon tablets are available as yellow, round shaped film coated tablets, debossed with "AC 414" on one side and plain on other side, in the following quantities: NDC 0832-1250-30Bottles ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Severe Anaphylactic and Anaphylactoid Reactions - Inform patients that severe anaphylactic and anaphylactoid ...
-
SPL UNCLASSIFIED SECTIONDistributed by - UPSHER-SMITH LABORATORIES, LLC - Maple Grove, MN 55369 - 200371 - Revised: 11/2021
-
MEDICATION GUIDERamelteon (ra-mel-tee-on) TabletsRead the Medication Guide that comes with ramelteon tablets before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place ...
-
PRINCIPAL DISPLAY PANEL - 8 mg Tablet Bottle LabelNDC 0832-1250-30 - Ramelteon - Tablets - 8 mg - PHARMACIST: Dispense the accompanying - Medication Guide to each patient. 30 Tablets - Rx only - UPSHER-SMITH
-
INGREDIENTS AND APPEARANCEProduct Information