Label: BRIMONIDINE- brimonidine tartrate solution/ drops
- NDC Code(s): 0781-7177-70, 0781-7177-75, 0781-7177-85
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BRIMONIDINE TARTRATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for BRIMONIDINE TARTRATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Brimonidine tartrate ophthalmic solution is an alpha adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular ...
-
2 DOSAGE AND ADMINISTRATION The recommended dose is one drop of brimonidine tartrate ophthalmic solution in the affected eye(s) three times daily, approximately 8 hours apart. Brimonidine tartrate ophthalmic solution may be ...
-
3 DOSAGE FORMS AND STRENGTHS Solution containing 1 mg/mL brimonidine tartrate.
-
4 CONTRAINDICATIONS 4.1 Neonates and Infants (under the age of 2 years) Brimonidine tartrate ophthalmic solution is contraindicated in neonates and infants (under the age of 2 years). 4.2 Hypersensitivity ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Potentiation of Vascular Insufficiency - Brimonidine tartrate ophthalmic solution may potentiate syndromes associated with vascular insufficiency. Brimonidine tartrate ophthalmic solution ...
-
6 ADVERSE REACTIONS 6.1 Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS 7.1 Antihypertensives/Cardiac Glycosides - Because brimonidine tartrate ophthalmic solution may reduce blood pressure, caution in using drugs such as antihypertensives and/or cardiac glycosides ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Teratogenicity studies have been performed in animals. Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in rats and days 6 through ...
-
10 OVERDOSAGE Very limited information exists on accidental ingestion of brimonidine in adults; the only adverse reaction reported to date has been hypotension. Symptoms of brimonidine overdose have been ...
-
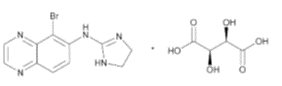
11 DESCRIPTION Brimonidine tartrate ophthalmic solution, 0.1%, sterile, is a relatively selective alpha-2 adrenergic receptor agonist (topical intraocular pressure lowering agent). The structural formula of ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Brimonidine tartrate ophthalmic solution is a relatively selective alpha-2 adrenergic receptor agonist with a peak ocular hypotensive effect occurring at two hours ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively ...
-
14 CLINICAL STUDIES Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Brimonidine tartrate has ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Brimonidine tartrate ophthalmic solution, 0.1% is supplied sterile, in a white LDPE plastic bottle with a natural LDPE dropper tip and a purple polypropylene cap as follows: 5 mL in 8 mL bottle ...
-
17 PATIENT COUNSELING INFORMATION Patients should be instructed that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common ...
-
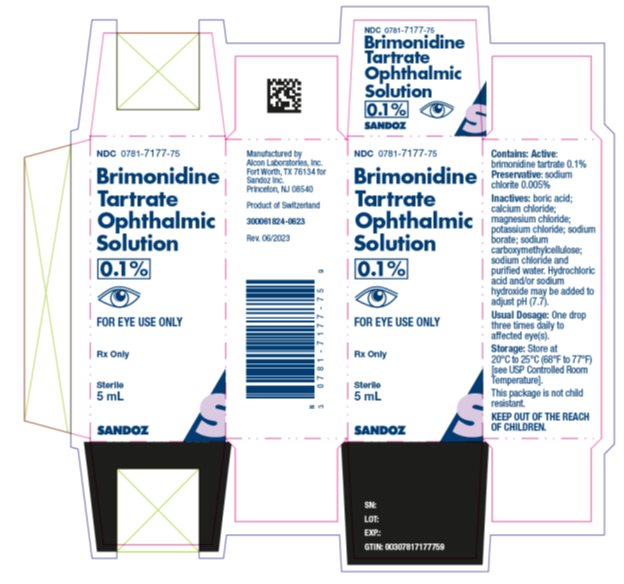
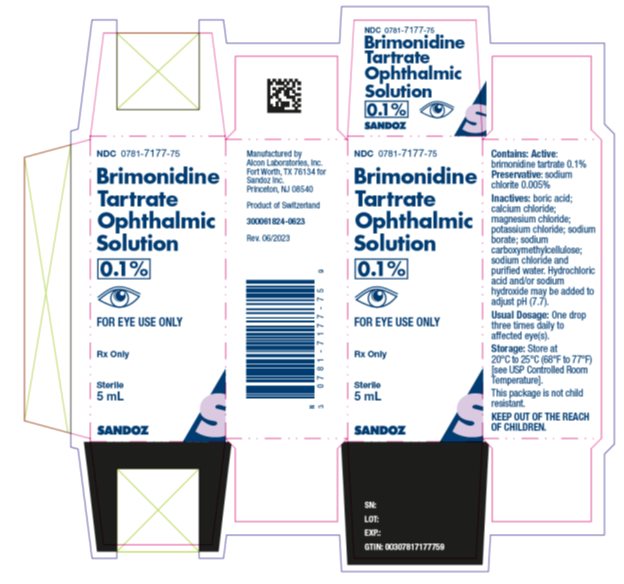
Package/Label Display PanelNDC 0781-7177-75 - Brimonidine - Tartrate - Ophthalmic - Solution - 0.1% FOR EYE USE ONLY. Rx Only - Sterile - 5 mL - SANDOZ
-
INGREDIENTS AND APPEARANCEProduct Information