Label: ISOSORBIDE DINITRATE tablet
- NDC Code(s): 0781-1556-01, 0781-1556-05, 0781-1556-10, 0781-1556-13, view more
- Packager: Sandoz Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
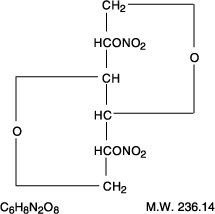
Rx OnlyDESCRIPTIONIsosorbide dinitrate (ISDN) is 1,4:3,6-dianhydro-D-glucitol 2,5-dinitrate, an organic nitrate whose structural formula is: The organic nitrates are vasodilators, active on both arteries and ...
-
CLINICAL PHARMACOLOGYThe principal pharmacological action of isosorbide dinitrate is relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins, especially the latter. Dilatation ...
-
INDICATIONS AND USAGEIsosorbide dinitrate tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of immediate-release oral isosorbide dinitrate is not ...
-
CONTRAINDICATIONSIsosorbide dinitrate tablets are contraindicated in patients who are allergic to isosorbide dinitrate or any of its ingredients. Do not use isosorbide dinitrate tablets in patients who are taking ...
-
WARNINGSAmplification of the vasodilatory effects of isosorbide dinitrate by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied ...
-
PRECAUTIONSGeneral - Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide dinitrate. This drug should therefore be used with caution in patients who may be ...
-
ADVERSE REACTIONSAdverse reactions to isosorbide dinitrate are generally dose-related, and almost all of these reactions are the result of isosorbide dinitrate’s activity as a vasodilator. Headache, which may be ...
-
OVERDOSAGEHemodynamic Effects - The ill effects of isosorbide dinitrate overdose are generally the results of isosorbide dinitrate’s capacity to induce vasodilatation, venous pooling, reduced cardiac ...
-
DOSAGE AND ADMINISTRATIONAs noted under CLINICAL PHARMACOLOGY, multiple-dose studies with ISDN and other nitrates have shown that maintenance of continuous 24-hour plasma levels results in refractory tolerance. Every ...
-
HOW SUPPLIEDIsosorbide dinitrate tablets, USP are supplied as: 5 mg: Round, pink, scored tablets, debossed GG 259 on one side and plain on the reverse side, and supplied as: NDC 0781-1635-01 bottles of ...
-
SPL UNCLASSIFIED SECTIONManufactured in India by - Sandoz Private Ltd., for - Sandoz Inc., Princeton, NJ 08540 - 46316474 - Rev. Feb 2023
-
5 mg LabelNDC 0781-1635-01 - Isosorbide - Dinitrate - Tablets, USP - 5 mg ORAL - Rx only - 100 Tablets - SANDOZ
-
10 mg LabelNDC 0781-1556-01 - Isosorbide - Dinitrate - Tablets, USP - 10 mg ORAL - Rx only - 100 Tablets - SANDOZ
-
20 mg LabelNDC 0781-1695-01 - Isosorbide - Dinitrate - Tablets, USP - 20 mg ORAL - Rx only - 100 Tablets - SANDOZ
-
10 mg Carton Label NDC 0781-8011-13 - Isosorbide - Dinitrate - Tablets, USP - 10 mg ORAL - Rx only - 100 Tablets - SANDOZ
-
20 mg Carton Label NDC 0781-8026-13 - Isosorbide - Dinitrate - Tablets, USP - 20 mg ORAL - Rx only - 100 Tablets - SANDOZ
-
INGREDIENTS AND APPEARANCEProduct Information