Label: COLESEVELAM HYDROCHLORIDE tablet, film coated
COLESEVELAM HYDROCHLORIDE for suspension
- NDC Code(s): 0713-0935-30, 0713-0936-81
- Packager: Cosette Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use COLESEVELAM HYDROCHLORIDE safely and effectively. See full prescribing information for COLESEVELAM HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Primary Hyperlipidemia - Colesevelam hydrochloride is indicated as an adjunct to diet and exercise to reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary ...
-
2 DOSAGE AND ADMINISTRATION2.1 Testing Prior to Initiation of Colesevelam Hydrochloride - Obtain lipid parameters, including triglyceride (TG) levels, before starting colesevelam hydrochloride. Colesevelam hydrochloride is ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 625 mg tablets are off-white, oval, film-coated and imprinted with "Sankyo" and "C01" on one side. For Oral Suspension: 3.75 gram packet containing a white to pale yellow powder with ...
-
4 CONTRAINDICATIONSColesevelam hydrochloride is contraindicated in patients with: Serum TG concentrations >500 mg/dL - [see - Warnings and Precautions (5.1)] History of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypertriglyceridemia and Pancreatitis - Colesevelam hydrochloride, like other bile acid sequestrants, can increase serum TG concentrations. Hypertriglyceridemia can cause acute ...
-
6 ADVERSE REACTIONSThe following important adverse reactions are described below and elsewhere in the labeling: Hypertriglyceridemia and Pancreatitis - [see - Warnings and Precautions (5.1) ...
-
7 DRUG INTERACTIONS7.1 Colesevelam Hydrochloride Drug Interactions that Decrease the Exposure of the Concomitant Medication - Table 4 includes a list of drugs that decrease exposure of the concomitant medication ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Colesevelam hydrochloride is not absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure to the drug ...
-
10 OVERDOSAGEColesevelam hydrochloride is not absorbed and the risk of systemic toxicity is low. Excessive doses of colesevelam hydrochloride may cause more severe local gastrointestinal effects (e.g. ...
-
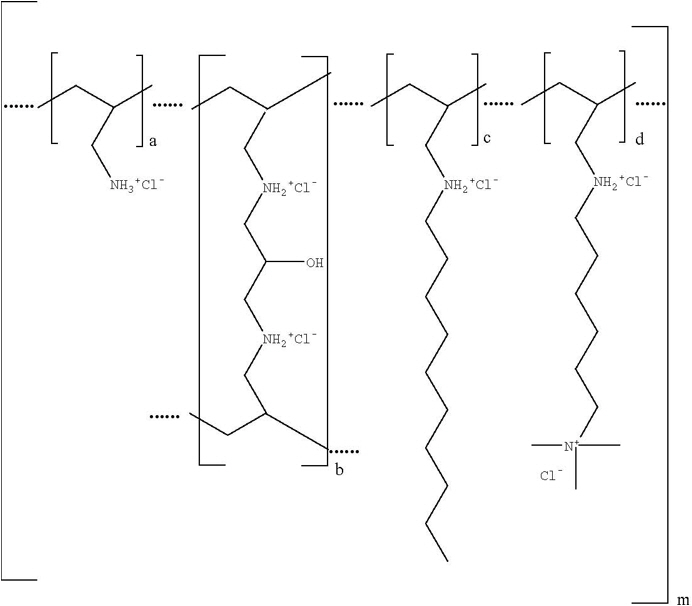
11 DESCRIPTIONColesevelam hydrochloride is a non-absorbed, polymeric, lipid-lowering and glucose-lowering agent for oral administration. Colesevelam hydrochloride is a high-capacity bile acid-binding ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Primary Hyperlipidemia: Colesevelam hydrochloride, the active pharmaceutical ingredient, is a non-absorbed, lipid-lowering polymer that binds bile acids in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - A 104-week carcinogenicity study with colesevelam hydrochloride was conducted in CD-1 mice, at oral dietary doses ...
-
14 CLINICAL STUDIES14.1 Primary Hyperlipidemia - Colesevelam hydrochloride reduces total cholesterol (TC), LDL-C, apolipoprotein B (Apo B), and non-high-density lipoprotein cholesterol (non-HDL-C) when administered ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGColesevelam hydrochloride 625 mg tablets are supplied as off-white, solid tablets imprinted with the word "Sankyo" and "C01" on one side and are available as follows: Bottles of 180 – NDC ...
-
17 PATIENT COUNSELING INFORMATIONHypertriglyceridemia and Pancreatitis - Inform patients that colesevelam hydrochloride may increase their serum triglycerides which can lead to hypertriglyceridemia and pancreatitis. Instruct ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Cosette Pharmaceuticals, Inc. South Plainfield, NJ 07080 - 8-COLCP1 Iss. 03/2022
-
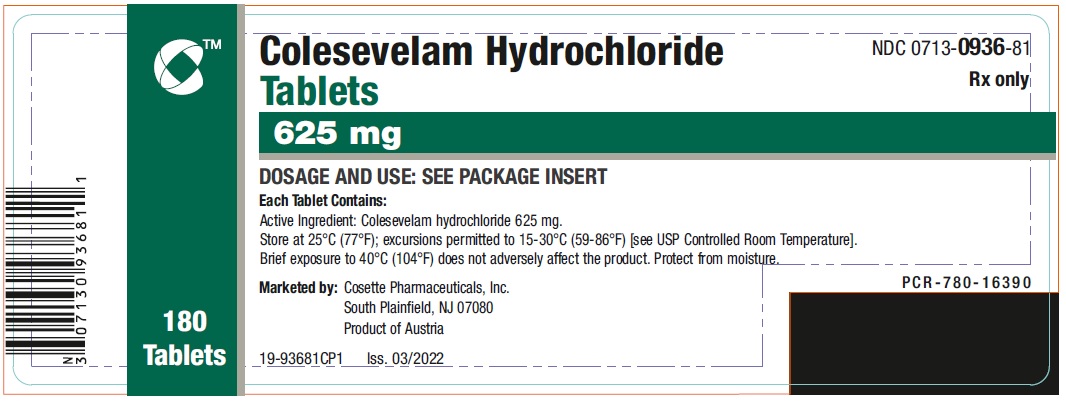
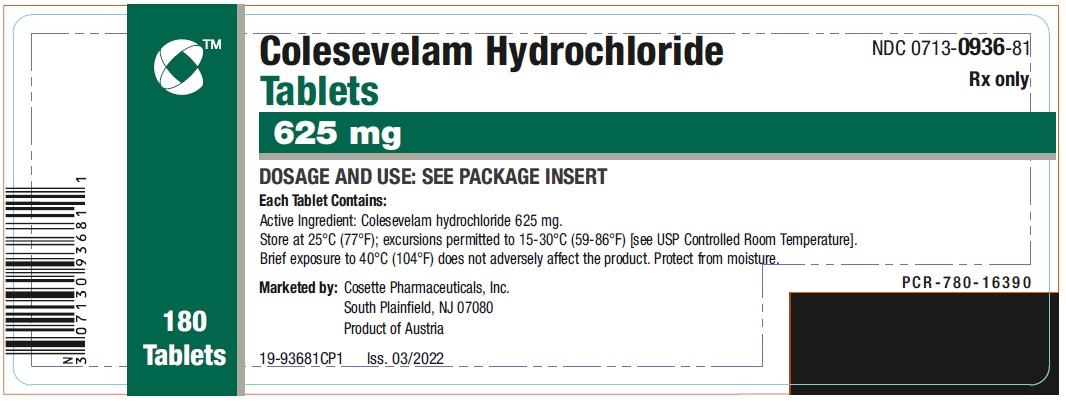
PRINCIPAL DISPLAY PANEL
NDC 0713-0936-81 - Colesevelam Hydrochloride - Tablets - 625 mg - 180 Tablets
-
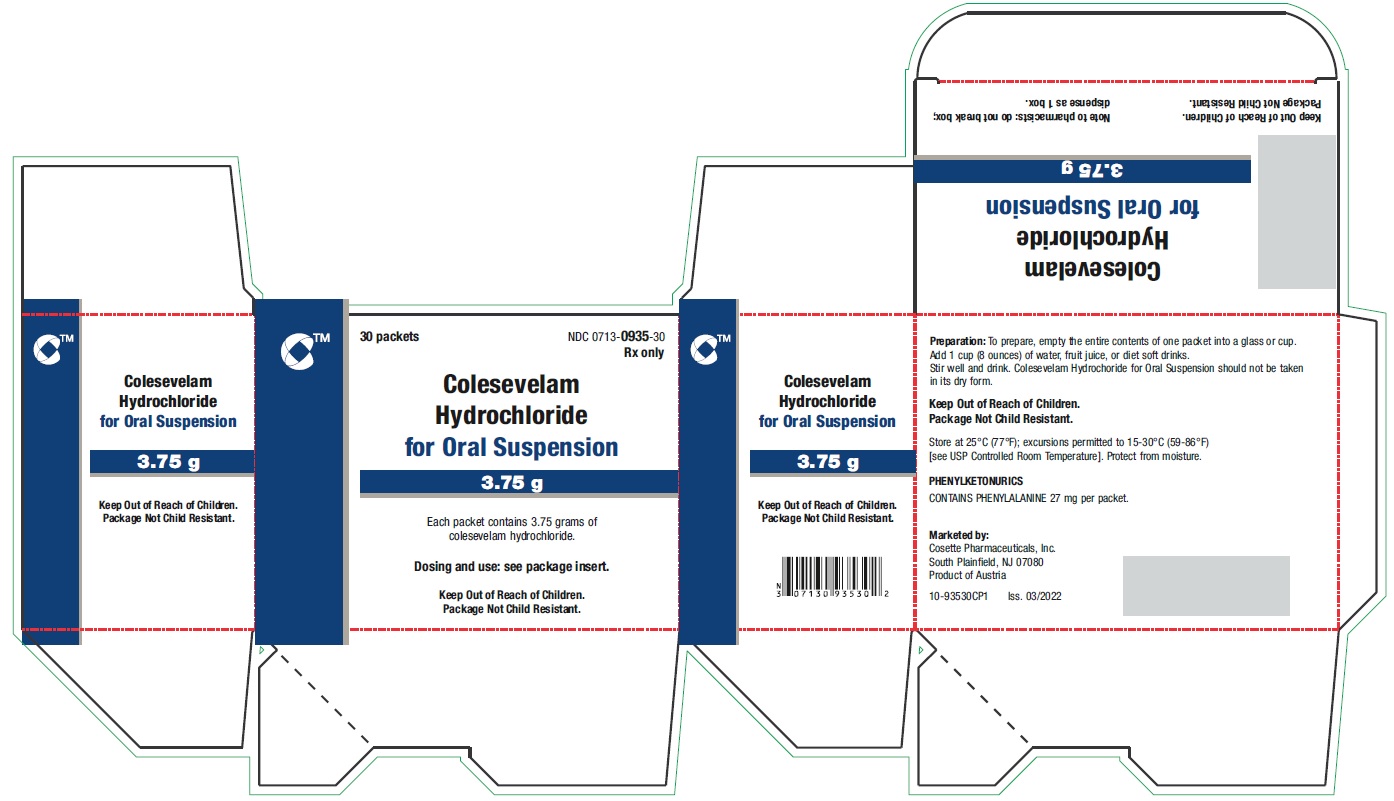
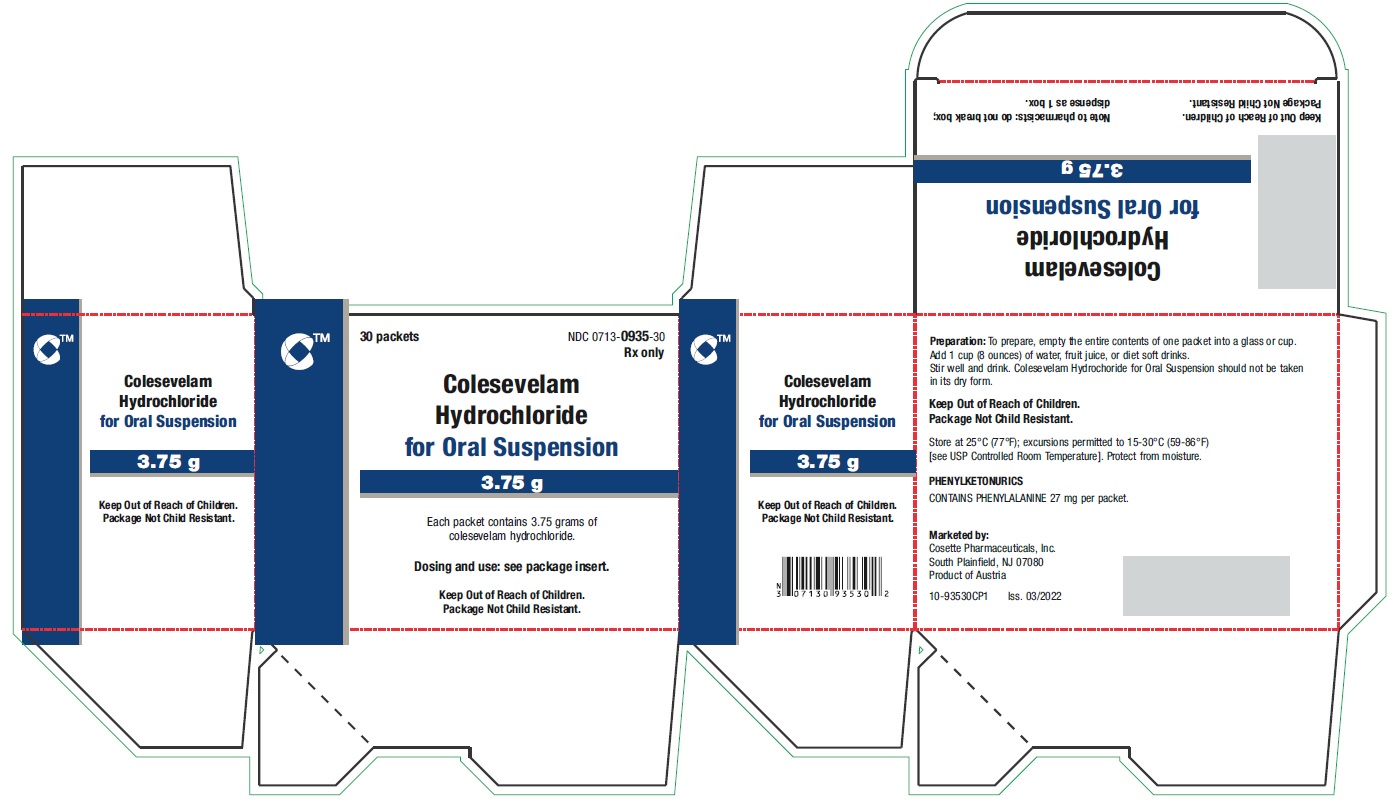
PRINCIPAL DISPLAY PANEL - 3.75 g Packet LabelTEAR HERE - No cutting required - Sugar-Free - This packet is contained within the - CARTON NDC 0713-0935-30 - Rx only - Colesevelam - Hydrochloride - for Oral Suspension - 3.75 ...
-
PRINCIPAL DISPLAY PANEL - 3.75 g Packet CartonNDC 0713-0935-30 - Rx only - 30 packets - Colesevelam - Hydrochloride - for Oral Suspension - 3.75 g - Each packet contains 3.75 grams of - colesevelam hydrochloride. Dosing and use ...
-
INGREDIENTS AND APPEARANCEProduct Information